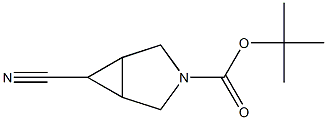
exo-3-Boc-6-cyano-3-azabicyclo[3.1.0]hexane synthesis
- Product Name:exo-3-Boc-6-cyano-3-azabicyclo[3.1.0]hexane
- CAS Number:871239-62-2
- Molecular formula:C11H16N2O2
- Molecular Weight:208.26
![tert-butyl (1R,5S,6r)-6-carbamoyl-3-azabicyclo[3.1.0]hexane-3-carboxylate](/CAS/20200401/GIF/1269429-62-0.gif)
1269429-62-0
6 suppliers
inquiry
![exo-3-Boc-6-cyano-3-azabicyclo[3.1.0]hexane](/CAS/20150408/GIF/871239-62-2.gif)
871239-62-2
23 suppliers
inquiry
Yield:871239-62-2 96%
Reaction Conditions:
with isocyanuric acid in N,N-dimethyl-formamide at 20; for 2 h;
Steps:
4.2 Step 2: Synthesis of tert-butyl (lR,5S,6r)-6-cyano-3-azabicyclo[3.1.0]hexane-3- carboxylate
To a 50-mL round-bottom flask was added tert-butyl (lS,5R)-6-carbamoyl-3- azabicyclo[3.1.0]hexane-3-carboxylate (1.00 g, 4.42 mmol, 1 equiv) and cyanuric acid (815 mg, 4.42 mmol, 1 equiv) in DMF (17 mL). The reaction was stirred for 2 hours at room (0434) temperature. After 2 hours, the reaction was diluted with water and extracted 3 times with i- PrOAc. The organic layer was then washed 2 times with water and then brine, dried over a2S04, and concentrated under vacuum to afford tert-butyl (lS,5R)-6-cyano-3-azabicyclo [3.1.0]hexane-3-carboxylate as a clear yellow oil which crystallized over time (879 mg, 96% yield).
References:
WO2018/162607,2018,A1 Location in patent:Paragraph 0243; 0244
![(1R,5S,6R)-3-(tert-butoxycarbonyl)-3-azabicyclo[3.1.0]hexane-6-carboxylic acid](/CAS/GIF/927679-54-7.gif)
927679-54-7
124 suppliers
$29.00/100mg
![exo-3-Boc-6-cyano-3-azabicyclo[3.1.0]hexane](/CAS/20150408/GIF/871239-62-2.gif)
871239-62-2
23 suppliers
inquiry
![(1R,5S,6r)-3-tert-butyl 6-ethyl 3-azabicyclo[3.1.0]hexane-3,6-dicarboxylate](/CAS/20210111/GIF/134575-37-4.gif)
134575-37-4
13 suppliers
inquiry
![exo-3-Boc-6-cyano-3-azabicyclo[3.1.0]hexane](/CAS/20150408/GIF/871239-62-2.gif)
871239-62-2
23 suppliers
inquiry