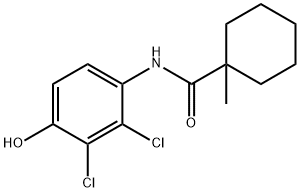
FENHEXAMID synthesis
- Product Name:FENHEXAMID
- CAS Number:126833-17-8
- Molecular formula:C14H17Cl2NO2
- Molecular Weight:302.2
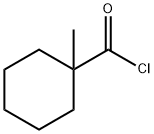
2890-61-1
34 suppliers
$134.31/250mgs:
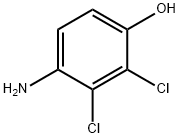
39183-17-0
70 suppliers
$22.00/100mg

126833-17-8
169 suppliers
$114.00/100mg
Yield:126833-17-8 90%
Reaction Conditions:
with triethylamine in ethyl acetate at 78; for 2.7 h;Large scale;Temperature;
Steps:
1.5
Step 5, adding 120 kg of 2,3-dichloro-4-hydroxyaniline, 113 kg of 1-methylcyclohexanoyl chloride and 800 kg of ethyl acetate to the finish product kettle, and heat to 78 ° C for reflux reaction, and add dropwise 72 kg of triethylamine during the reflux reaction. Triethylamine was added dropwise within 0.7 hours. Afterwards, reflux reaction was continued at 78 ° C for 2 hours. After the reaction was completed, the temperature was lowered to 25 ° C, and filtered under a negative pressure of -0.087 MPa for 10 minutes to obtain three. Ethylamine hydrochloride and mother liquor. Add 10kg of activated carbon to the mother liquor (activated carbon for decolorization and impurity removal), reflux at 78 ° C for 1 hour, filter under vacuum conditions of -0.087 MPa for 10 minutes, add 300 kg of water, atmospheric pressure at 78 ° C The ethyl acetate was recovered, and the ethyl acetate was recovered for 2.5 to 3 hours, and the obtained product was suspended in water, and centrifuged at 3200 rpm for 10 minutes at 35 ° C to obtain a crude product, which was dried at 80 ° C for 3 hours. Fenhexamid is obtained. The weight of fenhexamid is 185-188 kg, the content is 98%, and the yield is over 90%. A specific process for synthesizing fenhexamid from 2,3-dichloro-4-hydroxyaniline and 1-methylcyclohexyl chloride is shown in formula (5).
References:
Shanxi Hengrun Chemical Co., Ltd.;Fan Ming;Li Cangzhen;Chen Dunguo;Chao Liumin;Wang Jianqiao;Yin Weixin CN108689875, 2018, A Location in patent:Paragraph 0053; 0063-0064; 0071; 0078
576-24-9
252 suppliers
$14.00/1g

126833-17-8
169 suppliers
$114.00/100mg
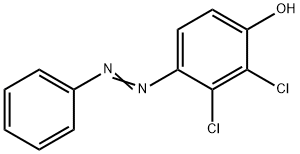
201656-71-5
0 suppliers
inquiry

126833-17-8
169 suppliers
$114.00/100mg