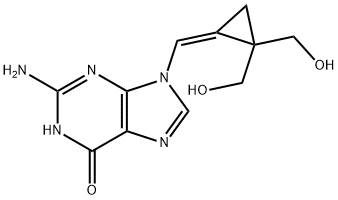
Filociclovir synthesis
- Product Name:Filociclovir
- CAS Number:632325-71-4
- Molecular formula:C11H13N5O3
- Molecular Weight:263.25
![1,1-Cyclopropanedimethanol, 2-[(2-amino-6-chloro-9H-purin-9-yl)methylene]-, (2Z)-](/CAS/20210305/GIF/632325-69-0.gif)
632325-69-0
0 suppliers
inquiry

632325-71-4
8 suppliers
inquiry
Yield:632325-71-4 89%
Reaction Conditions:
Stage #1: (Z)-2-amino-6-chloro-9-{[2,2-bis(hydroxymethyl)cyclopropylidene]methyl}purinewith formic acid at 80; for 4 h;
Stage #2: with ammonia in methanol at 0; for 4 h;
Stage #3: with methanol for 2 h;Heating / reflux;
Steps:
11 (Z)-9-{[2,2-Bis-(hydroxymethyl)cyclopropylidene]methyl}-guanine (1c)
EXAMPLE 11 (Z)-9-{[2,2-Bis-(hydroxymethyl)cyclopropylidene]methyl}-guanine (1c) The solution of the Z-isomer 1b (100 mg, 0.36 mmol) from Example 10 in formic acid (95-97%, 8 mL) was heated at 80° C. with stirring for 4 h. After cooling, the formic acid was evaporated in vacuo and the crude product was dissolved in methanol (30 mL). A precipitated white solid was stirred in methanolic ammonia (20%, 10 mL) at 0° C. for 4 h. After evaporation of volatile components, a suspension of the residue in methanol (100 mL) was refluxed for 2 h. The mixture was kept overnight at 0° C. to give product 1c (83 mg, 89%). Mp. >300° C. UV max (ethanol) 271 nm (ε 11,500), 231 nm (ε 26,400). 1H NMR (CD3SOCD3, 400 MHz) δ 1.29 (s, 2H, H3'), 3.48, 3.63 and 3.49, 3.62 (2AB, 4H, 2J=10.8 and 11.2 Hz, 4H, 2J=10.8 and 11.2, H5')', 4.99 (t, 2H, J=5.6 Hz, OH), 6.52 (s, 2H, NH2), 7.07 (s, 1H, H1'), 8.41 (s, 1H, H8), 10.64 (s, 1H, NH). 13C NMR (CD3SOCD3, 100 MHz) ppm 11.52 (C3'), 31.26 (C4'), 62.75 (C5'), 110.98 (C1'), 116.92 (C2'), 118.08 (C5), 135.13 (C8), 150.29 (C4), 154.56 (C2), 157.38 (C6). ESI-MS (MeOH+NaCl) 264 (M+H, 5.1), 286 (M+Na, 100.0), 549 (2M+Na, 41.1). Calculated for C11H13N5O3: C, 50.19; H, 4.98; N, 26.60. Found: C, 50.06; H, 5.09; N, 26.48.
References:
US2005/113393,2005,A1 Location in patent:Page/Page column 9
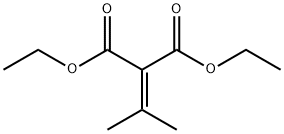
6802-75-1
155 suppliers
$9.00/5g

632325-71-4
8 suppliers
inquiry
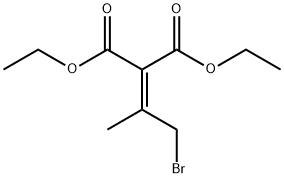
13830-91-6
1 suppliers
inquiry

632325-71-4
8 suppliers
inquiry
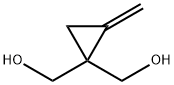
33475-19-3
0 suppliers
inquiry

632325-71-4
8 suppliers
inquiry
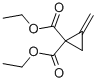
106352-19-6
20 suppliers
inquiry

632325-71-4
8 suppliers
inquiry