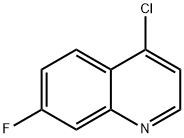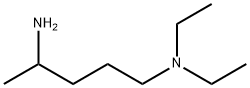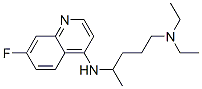
fluoroquine synthesis
- Product Name:fluoroquine
- CAS Number:442-96-6
- Molecular formula:
- Molecular Weight:0
Yield: 48%
Reaction Conditions:
in neat (no solvent) at 100 - 150; for 7 h;
Steps:
4.B Step B: Synthesis of N1.N1-diethyl-N4-(7-fluoroquinolin-4-yl)pentane-1 ,4-diamine
An oven dried 10 mL vial was charged with 4-chloro-7-fiuoroquinoline(0.5 g, 2.5 mmol). Neat 2-amino-5-diethylaminopentane (1.2 mL, 6.2 mmol) was added and the contents were heated at 100°C for 2 h and at 150°C for 5 h. The reaction mixture was purified on column chromatography on alumina (10 % - 40 % of ethyl acetate in n-hexanes) to yield N1,N1-diethyl-N4-(7-fluoroquinolin-4-yl)pentane- 1,4- diamine (0.4 g, 48 %) as white solid.0.5 (20 % of ethyl acetate in w-hexanes, alumina, UVR/active)Thermo-MS (ESI) m/z (M+ H) Cald for Ci8H26FN3: 304.42, found: 304.4 δ 8.47 (d, J = 4 Hz, 1H), 7.67 (dd, Ji = 3.2 Hz, J2= 5.2 Hz, 1H), 7.54 (dd, Ji = 4 Hz, J2= 8 Hz, 1H), 7.19-7.05 ^-NMR (500 MHz, CDC13) (m, 1H), 6.37 (d, J = 8 Hz, 1H), 5.15 (d, J = 4 Hz, 1H),3.75-3.61 (m, 1H), 2.48 (q, J= 8 Hz, 4H), 2.41 (t, J = 8 Hz, 2H), 1.71-.147 (m, 4 H), 1.28 (d, J = 8 Hz, 3 H), 0.97 (t, J = 8 Hz, 6 H)δ 163.91, 161.44, 151.86, 150.02, 149.90, 149.35,1C-NMR (100 MHz,122.33, 122.23, 115.83, 113.93, 113.93, 113.68, 113.07, CDCI3) 112.87, 98.56, 52.4, 48.17, 46.64, 34.35, 23.74, 20.01,11.28
References:
UNIVERSITY OF ROCHESTER;BOECKMAN, Robert;BOYCE, Brendan;XIAO, Lifeng;YAO, Zhenqiang;EBETINO, Frank, H. WO2017/79260, 2017, A1 Location in patent:Page/Page column 85; 86
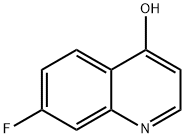
391-83-3
52 suppliers
$45.00/50mg

442-96-6
2 suppliers
inquiry
372-19-0
320 suppliers
$10.00/1g

442-96-6
2 suppliers
inquiry