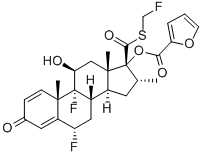
FLUTICASONE FUROATE synthesis
- Product Name:FLUTICASONE FUROATE
- CAS Number:397864-44-7
- Molecular formula:C27H29F3O6S
- Molecular Weight:538.58
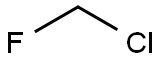
397864-40-3
26 suppliers
inquiry
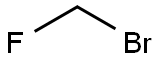
373-52-4
126 suppliers
inquiry

397864-44-7
184 suppliers
$25.00/1mg
Yield:397864-44-7 99.3%
Reaction Conditions:
in butanone at 0 - 22;
Steps:
1.3
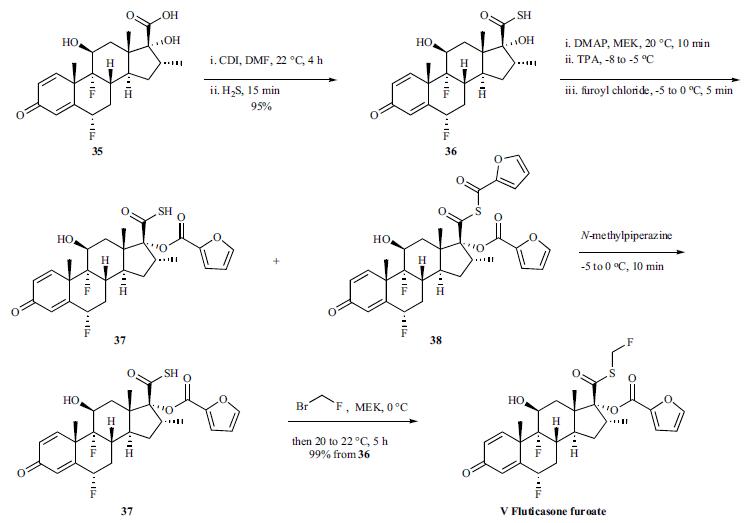
Step 3: A solution of bromofluoromethane (3.28 g, 1.2 eq wrt the thioacid) in MEK (10 ml, 32.8% w/v) was then added rapidly as a single charge at 0° C. The solution was then warmed rapidly to 20-22° C. and stirred for a total of 5 hours at 20-22° C. (HPLC indicated that no thioacid furoate (compound of formula (III)) remained).The reaction mixture was then diluted with MIBK (230 ml) and washed subsequently with aqueous 2M hydrochloric acid (2×50 ml); water (1×50 ml); aqueous potassium carbonate (4% w/v, 1×30 ml) and then water (1×30 ml). The final organic phase was then concentrated under reduced pressure to give a fine off-white solid (13.01 g, 99.3% theoretical yield after correction for MIBK, 97.43% purity).
References:
GLAXO GROUP LIMITED US2009/118495, 2009, A1 Location in patent:Page/Page column 4
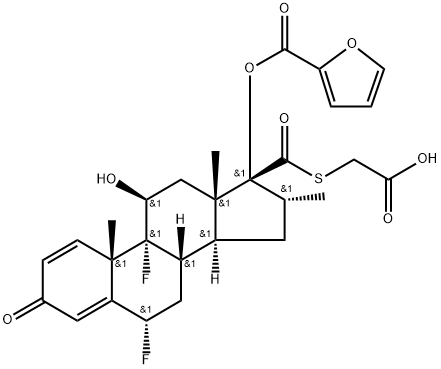
1351451-83-6
1 suppliers
inquiry

397864-44-7
184 suppliers
$25.00/1mg

397864-40-3
26 suppliers
inquiry
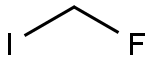
373-53-5
61 suppliers
$165.00/1 g

397864-44-7
184 suppliers
$25.00/1mg
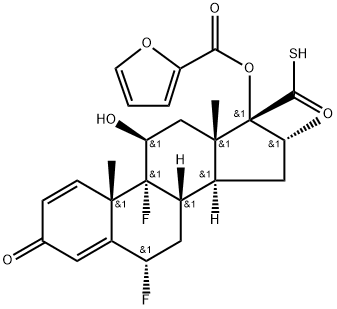
![Androsta-1,4-diene-17-carbothioic acid, 6,9-difluoro-17-[(2-furanylcarbonyl)oxy]-11-hydroxy-16-methyl-3-oxo-, anhydrosulfide with 2-furancarbothioic acid, (6α,11β,16α,17α)-](/CAS/20211123/GIF/397864-41-4.gif)