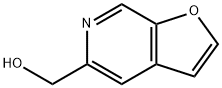
Furo[2,3-c]pyridine-5-methanol (9CI) synthesis
- Product Name:Furo[2,3-c]pyridine-5-methanol (9CI)
- CAS Number:478148-60-6
- Molecular formula:C8H7NO2
- Molecular Weight:149.15
![Furo[2,3-c]pyridine-5-methanol, 2-(trimethylsilyl)-](/CAS/20210305/GIF/478148-59-3.gif)
478148-59-3
![Furo[2,3-c]pyridine-5-methanol (9CI)](/CAS/GIF/478148-60-6.gif)
478148-60-6
General procedure for the synthesis of furo[2,3-c]pyridin-5-ylmethanol from the compound (CAS: 478148-59-3): C14 (770 mg, 3.48 mmol) was dissolved in 10 mL of methanol. A 2 N sodium hydroxide solution (3 mL, 6 mmol) was added to the solution and the reaction mixture was stirred at room temperature for 1.5 hours. After completion of the reaction, the solution was concentrated to dryness under vacuum. Water (20 mL) was added to the residue and extracted with dichloromethane (4 x 10 mL). The organic layers were combined, dried with anhydrous potassium carbonate, filtered, and concentrated under vacuum to give furo[2,3-c]pyridin-5-ylmethanol (C16) as a white solid in 90% yield. Elemental analysis (C8H7NO2) Calculated values: C, 64.42; H, 4.73; N, 9.39. Measured values: C, 64.60; H, 4.56; N, 9.44.
![Furo[2,3-c]pyridine-5-methanol, 5-acetate](/CAS/20200515/GIF/478149-20-1.gif)
478149-20-1
0 suppliers
inquiry
![Furo[2,3-c]pyridine-5-methanol (9CI)](/CAS/GIF/478148-60-6.gif)
478148-60-6
53 suppliers
$240.00/250mg
Yield:478148-60-6 92%
Reaction Conditions:
with methanol;potassium carbonate at 20;
Steps:
59.a.8
a-8) Preparation of furo[2,3-c]pyridin-5-yl methanol Furo[2,3-c]pyridin-5-yl methyl acetate (722 mg, 3.78 mmol) was dissolved in methanol (19 mL), added potassium carbonate (1.04 g, 7.56 mmol) at room temperature, and the mixture was stirred at room temperature overnight. The reaction solution was added 1N-aqueous solution of hydrochloric acid under ice-cold conditions, and extracted with chloroform. The organic layer was dried over anhydrous sodium sulfate, and concentrated in vacuo. The obtained residue was purified by silica-gel column chromatography (hexane/ethyl acetate), and the title compound (521 mg (yield 92%)) was obtained as a yellow oil. 1H-NMR (CDCl3) δ: 3.68 (1H, s), 4.85 (2H, s), 6.79 (1H, s), 7.54 (1H, s), 7.79 (1H, s), 8.83 (1H, s).
References:
US2010/280013,2010,A1 Location in patent:Page/Page column 52-53
![Furo[2,3-c]pyridine-5-methanol, 2-(trimethylsilyl)-](/CAS/20210305/GIF/478148-59-3.gif)
478148-59-3
0 suppliers
inquiry
![Furo[2,3-c]pyridine-5-methanol (9CI)](/CAS/GIF/478148-60-6.gif)
478148-60-6
53 suppliers
$240.00/250mg
![(7-Chloro-furo[2,3-c]pyridin-5-yl)-methanol](/CAS/20180808/GIF/208519-39-5.gif)
208519-39-5
2 suppliers
inquiry
![Furo[2,3-c]pyridine-5-methanol (9CI)](/CAS/GIF/478148-60-6.gif)
478148-60-6
53 suppliers
$240.00/250mg
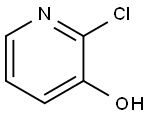
6636-78-8
435 suppliers
$5.00/1g
![Furo[2,3-c]pyridine-5-methanol (9CI)](/CAS/GIF/478148-60-6.gif)
478148-60-6
53 suppliers
$240.00/250mg
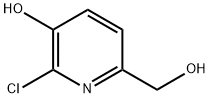
208519-41-9
61 suppliers
$45.00/50mg
![Furo[2,3-c]pyridine-5-methanol (9CI)](/CAS/GIF/478148-60-6.gif)
478148-60-6
53 suppliers
$240.00/250mg