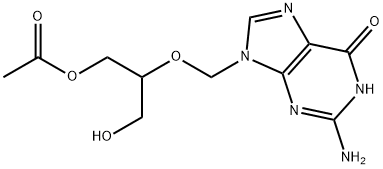
Ganciclovir Mono-O-acetate synthesis
- Product Name:Ganciclovir Mono-O-acetate
- CAS Number:88110-89-8
- Molecular formula:C11H15N5O5
- Molecular Weight:297.27
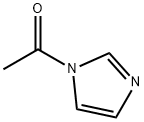
2466-76-4
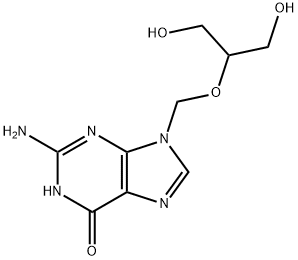
82410-32-0

88110-89-8
The general procedure for the synthesis of 2-((2-amino-6-oxo-1H-purin-9(6H)-yl)methoxy)-3-hydroxypropyl acetate from 1-acetylimidazole and 2-amino-9-(((1,3-dihydroxypropan-2- yl)oxy)methyl)-1H-purin-6(9H)-one is as follows: 500.0 g (1.96 mol, 1.0 equiv) of ganciclovir, 244.4 g (2.35 mol, 1.2 eq.) of trimethyl borate and 7.5 kg of toluene were added to the reaction vessel and heated to reflux for 5 hours. The progress of the reaction was monitored by TLC to confirm the complete reaction of the ingredients. The reaction mixture was cooled to room temperature and 396.7 g (3.93 mol, 2.0 eq.) of triethylamine was added, followed by 323.7 g (2.94 mol, 1.5 eq.) of 1-acetylimidazole, and stirred for 6 hours at room temperature. The ratio of monoacetyl ganciclovir to N,O-diacetyl ganciclovir was analyzed by HPLC to be 94:4. The reaction mixture was cooled to 0-10 °C and 2.0 kg of methanol was slowly added dropwise followed by stirring for 2 h at room temperature. The solvent was removed by concentration under reduced pressure, 3.0 kg of ethyl acetate was added, the organic layer was washed with 500 g of water and the aqueous layer was extracted with 500 g of ethyl acetate. The organic layers were combined, concentrated under reduced pressure and recrystallized by ethanol to give 440.9 g of colorless crystal product in 75.7% yield and 99.2% purity.
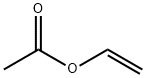
108-05-4
593 suppliers
$4.00/1000g

82410-32-0
613 suppliers
$5.00/100mg

88110-89-8
141 suppliers
$45.00/100mg
Yield: 75.2%
Reaction Conditions:
Stage #1:ganciclovir with triethyl borate in 2-methyltetrahydrofuran for 3 h;Reflux;Large scale;
Stage #2:vinyl acetate with triethylamine at 20; for 6 h;Large scale;
Stage #3: in ethanol at 0 - 20; for 3 h;Large scale;Reagent/catalyst;Solvent;
Steps:
3
500.0 g (1.96 mol, 1.0 eq) of ganciclovir, 314.7 g (2.15 mol, 1.1 eq) of triethyl borate,5.0 kg of 2-methyltetrahydrofuran was added to the reaction vessel, and heated to reflux for 3 hours.The TLC controlled raw material reacted completely to room temperature.595.0 g (5.88 mol, 3.0 eq) of triethylamine was added, and then 421.8 g (4.9 mol, 2.5 eq) of vinyl acetate was added, and the mixture was stirred at room temperature for 6 hours.The ratio of monoacetyl ganciclovirand N,O-diacetyl ganciclovir in the HPLC was 93/5, and the temperature was lowered by 0-10 °C.2.0 kg of ethanol was added dropwise, and the mixture was stirred at room temperature for 3 hours, and the solvent was concentrated under reduced pressure.It was washed once with 500 g of water, and the layers were separated.The ethanol was recrystallized to obtain 438.0 g of colorless crystals, the yield was 75.2%, and the purity was 99.1%.
References:
Anhui Haikang Pharmaceutical Co., Ltd.;Zhang Xiaoshun CN108383840, 2018, A Location in patent:Paragraph 0029; 0035; 0036; 0038; 0052

2466-76-4
364 suppliers
$13.00/5g

82410-32-0
613 suppliers
$5.00/100mg

88110-89-8
141 suppliers
$45.00/100mg
![N-[9-[[2-(Acetyloxy)-1-[(acetyloxy)methyl]ethoxy]methyl]-6,9-dihydro-6-oxo-1H-purin-2-yl]acetamide](/CAS/GIF/86357-14-4.gif)
86357-14-4
154 suppliers
$19.00/5g

88110-89-8
141 suppliers
$45.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

88110-89-8
141 suppliers
$45.00/100mg
1445-45-0
359 suppliers
$10.00/5mL

82410-32-0
613 suppliers
$5.00/100mg

88110-89-8
141 suppliers
$45.00/100mg