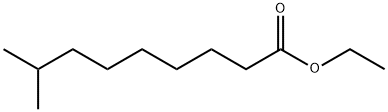
GFHUSRSVULITGF-UHFFFAOYSA-N synthesis
- Product Name:GFHUSRSVULITGF-UHFFFAOYSA-N
- CAS Number:905589-94-8
- Molecular formula:C12H24O2
- Molecular Weight:200.32
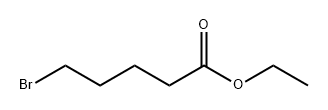
14660-52-7
233 suppliers
$6.00/5g

905589-94-8
5 suppliers
inquiry
Yield:905589-94-8 74.7%
Reaction Conditions:
Stage #1: i-pentyl bromidewith magnesium in tetrahydrofuran at 20; for 3 h;
Stage #2: ethyl 5-bromovaleratewith 1-methyl-pyrrolidin-2-one;copper(l) chloride in tetrahydrofuran at 0; for 2.41667 h;Product distribution / selectivity;
Steps:
1
[Example 1] Synthesis of 8-methylnonanoic acid (example of cross coupling method); Under an argon atmosphere, Mg turnings (6.12 g, 252 mmol) was suspended in THF (10 ml) . 200 mg from isopentyl bromide (34.6 g, 229 mmol) was added at room temperature, and exothermic heat and foaming were confirmed. THF (50 ml) was added, and a solution of whole remainder of isopentyl bromide in THF (65 ml) was slowly added dropwise at room temperature over 1 hr and the mixture was stirred for 2 hrs. At this time, mild refluxing state was achieved. The reaction solution was filtered through cotton plug while washing with THF to give a solution (total amount 180 ml) of isopentylmagnesium bromide in THF.Under an argon atmosphere, copper (I) chloride (426 mg, 4.30 mmol) was dissolved in NMP (55.2 ml, 575 mmol). The EPO
References:
WO2006/88239,2006,A1 Location in patent:Page/Page column 34-35
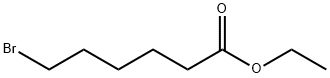
25542-62-5
255 suppliers
$6.00/5g
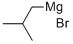
926-62-5
166 suppliers
$140.00/250g

905589-94-8
5 suppliers
inquiry