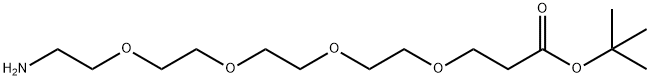
H2N-PEG4-tBu synthesis
- Product Name:H2N-PEG4-tBu
- CAS Number:581065-95-4
- Molecular formula:C15H31NO6
- Molecular Weight:321.41
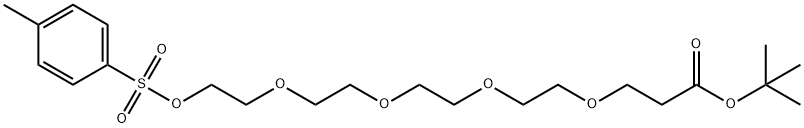
581065-94-3

581065-95-4
The general procedure for the synthesis of tert-butyl 1-amino-3,6,9,12-tetraoxapentadecan-15-oate from 1-(p-toluenesulfonyloxy)-3,6,9,12-tetraoxapentadecan-15-oate is as follows: 150 g of the sulfonated crude product was dissolved in 200 mL of methylene chloride, followed by the addition of 100 g of ammonium hydroxide and 10 g of tetrabutyl ammonium bromide. The reaction mixture was warmed to 40 °C with continuous stirring for 24 hours. Upon completion of the reaction, the solvent was removed by rotary evaporation. To the residue, 3 M hydrochloric acid solution was added and the pH was adjusted to 3. The organic phase was separated by extracting twice with 300 mL of dichloromethane. The pH of the aqueous phase was then adjusted to 9 with sodium hydroxide solution and extracted three times with 400 mL of dichloromethane. The organic phases were combined, dried with anhydrous sodium sulfate, filtered and the solvent was removed by rotary evaporation to give 50 g of aminocapped product. The structure of the product was confirmed by NMR.

581065-94-3
66 suppliers
$135.00/1G

581065-95-4
165 suppliers
$42.00/100mg
Yield:581065-95-4 50 g
Reaction Conditions:
with ammonium hydroxide;tetrabutylammomium bromide in dichloromethane at 40; for 24 h;
Steps:
1.3
The 150 g of the sulfonylated crude product obtained in step (2) was added to 200 ml of methylene chloride,Then add 100g ammonium hydroxide,10g tetrabutylammonium bromide,Warmed to 40 ,Stir for 24h.The reaction is over,Spin dry,Add 3M hydrochloric acid,Adjust PH = 3,With 300ml of dichloromethane,Extract 2 times,Then use sodium hydroxide solution to adjust PH = 9,Then extracted three times with 400ml of dichloromethane,Dried over anhydrous sodium sulfate,Spin dry,50 g of amino-terminated product was obtained.NMR data are as follows:
References:
Hunan Huateng Pharmaceutical Co., Ltd.;Deng Zeping;Li Hu;Cheng Jia CN107235848, 2017, A Location in patent:Paragraph 0021
![Bis[2-(2-hydroxyethoxy)ethyl] ether](/CAS/GIF/112-60-7.gif)
112-60-7
372 suppliers
$7.00/25g

581065-95-4
165 suppliers
$42.00/100mg
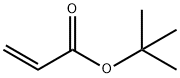
1663-39-4
260 suppliers
$10.00/5g

581065-95-4
165 suppliers
$42.00/100mg
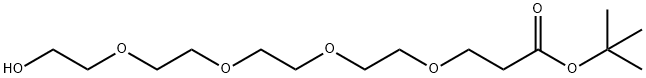
518044-32-1
143 suppliers
$17.00/100mg

581065-95-4
165 suppliers
$42.00/100mg