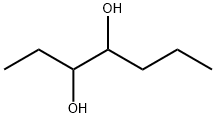
heptane-3,4-diol synthesis
- Product Name:heptane-3,4-diol
- CAS Number:62593-33-3
- Molecular formula:C7H16O2
- Molecular Weight:132.2
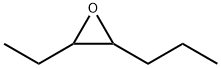
53897-32-8
4 suppliers
inquiry

62593-33-3
3 suppliers
inquiry
Yield:62593-33-3 90.1%
Reaction Conditions:
with water at 125; under 16501.7 Torr;
Steps:
8
In a 100 ml jacketed stainless steel tubular reactor, 30 ml of a halogen-substituted macroporous polystyrene quaternary ammonium salt type anion exchange resin catalyst having a structure represented by the general formula (3) was charged, and the upper and lower ends of the catalyst bed Filled with inert ceramic ball.The temperature of the reactor is controlled by an external circulation type heat transfer oil, which is controlled by a back pressure valve mounted on the outlet line. The reaction material is fed from the bottom of the reactor through the metering pump and flows through the catalyst bed from the top of the reactor , Cooled by the cooler and flowed into the reaction product tank.Time sampling, gas chromatography analysis reaction product composition.The reaction raw materials used in Examples 1 to 10 are shown in Table 1. The catalyst used has the structure of the general formula (3), wherein X and y are the same as those of the reaction formula, wherein the reaction temperature, pressure, liquid hourly space velocity and catalyst type change affect the reaction. , R5, R6, R7Group, resin crosslinking degree, resin alkali exchange capacity (dry basis) in Table 2, the reaction process conditions and reaction results in Table 3.The catalyst represented by the general formula (3) is prepared by preparing halogenated styrene and diene as reaction materials, and the reaction equation is as follows:
References:
CN106478375,2017,A Location in patent:Paragraph 0027; 0029; 0030; 0036
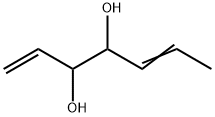
51945-98-3
1 suppliers
inquiry

62593-33-3
3 suppliers
inquiry

74328-37-3
0 suppliers
inquiry

62593-33-3
3 suppliers
inquiry
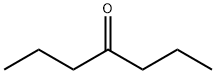
123-19-3
191 suppliers
$16.00/25mL

62593-33-3
3 suppliers
inquiry
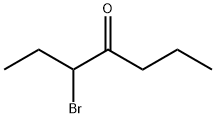
42330-10-9
16 suppliers
$62.89/1g

62593-33-3
3 suppliers
inquiry