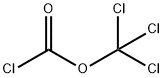
HEXYL CHLOROFORMATE synthesis
- Product Name:HEXYL CHLOROFORMATE
- CAS Number:6092-54-2
- Molecular formula:C7H13ClO2
- Molecular Weight:164.63
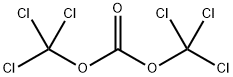
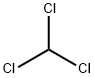
32315-10-9
441 suppliers
$10.00/1g

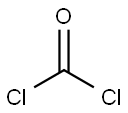
111-27-3
547 suppliers
$5.00/25g

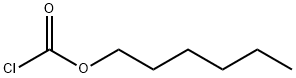
6092-54-2
300 suppliers
$6.00/1g
Yield:-
Reaction Conditions:
with pyridine in dichloromethane at 0 - 20; for 2 h;
Steps:
1.2 Example 1; Hexyl N-[2-[3-chloro-4-(2-methylbenzoyl)-phenylamino]phenyl]carbamate (Compound 101); General procedure: 2
To a stirred solution of an alcohol (1.0 mmol), with the general formula IV, in CH2Cl2 (3.0 ml) were added BTC (0.40 mmol) and pyridine (1.0 mmol) in CH2Cl2 (3.0 ml) at 0 °C. The reaction mixture was warmed to room temperature and stirred for 2 h. The solvent was removed in vacuo at 30 °C and the residue dissolved in EtOAc (10 ml) and stirred for 30 min. The precipitate was filtered off and the solvent removed in vacuo at 30 °C to give the crude chloroformate, with the general formula III. CH2Cl2 (5.0 ml) was added and the solution cooled to 0 °C. An amine (0.50 mmol), with the general formula II, and K2CO3 (2.0 mmol) were added and the reaction mixture was stirred for 24 h at room temperature. The reaction mixture was poured into water and extracted with CH2Cl2 or Et2O. The organic extracts were washed with brine, dried (Na2SO4), filtered and concentrated in vacuo to afford the crude product. The crude product was purified by chromatography to give the title compound. Starting compound II: 4-(2-Aminophenylamino)-2-chloro-2'-methylbenzophenone Starting compound VI: 1-Hexanol Purification: Chromatography using CH2Cl2 as eluent13C NMR (CDCl3): δ 196.9, 154.5, 149.4, 139.3, 138.0, 135.2, 133.7, 133.5, 131.4, 131.0, 130.7, 129.8, 129.0, 126.9, 126.0, 125.5, 125.0, 121.9, 116.3, 112.5, 66.1, 31.6, 29.0, 25.6, 22.7, 20.6, 14.2
References:
LEO PHARMA A/S EP1202959, 2004, B1 Location in patent:Page 13-14; 15-16