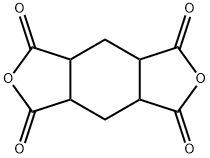
1,2,4,5-Cyclohexanetetracarboxylic Dianhydride synthesis
- Product Name:1,2,4,5-Cyclohexanetetracarboxylic Dianhydride
- CAS Number:2754-41-8
- Molecular formula:C10H8O6
- Molecular Weight:224.17
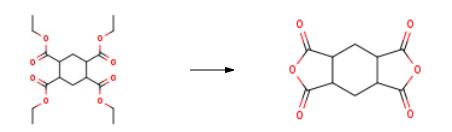
In a 100 mL three-necked flask equipped with a thermometer, a magnetic stirrer, a water separator, and a condenser tube was added 9.64 g of hydrogenatedEthylbenzene tetracarboxylate, 1mL of concentrated sulfuric acid, 5mL of acetic acid and 50mL of xylene were heated to 130°C. During the reaction, the reaction temperature was maintained at reflux, and the reaction continuously removed water for 5 hours. , cooling down to room temperature, suction filtration, filter cake washed with acetone, dried at room temperature under reduced pressure to give white solid 1,2,4,5-cyclohexane tetracarboxylic acid anhydride 4.80g, yield: 82.8%.
15383-49-0
86 suppliers
$6.00/250mg

2754-41-8
204 suppliers
$5.00/1g
Yield:2754-41-8 97.5%
Reaction Conditions:
with acetic anhydride for 1 h;Inert atmosphere;Reflux;
Steps:
2
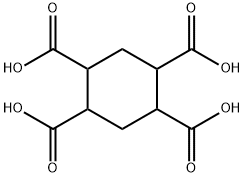
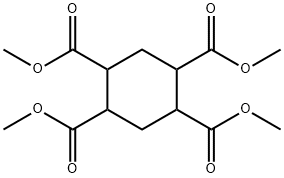
39.2 g of hydrogenated pyromellitic acid (0.15 mol) having a purity of 99.50% obtained in the step 1 was added to 500 mL four-necked flask , meanwhile adding 120 g of acetic anhydride (1.18 mol) and heating to reflux under a nitrogen atmosphere ,to carry out Dehydration reaction for 1h. [0026] After the reaction was completed, the solution was cooled to precipitate white crystals and filtered. The filter cake was dried under reduced pressure to obtain 32.5 g of white solid hydrogenated pyromellitic dianhydride. The yield was 97.5% and the purity of GC was 99.85%.
References:
Changzhou Sunshine Pharmaceutical Co., Ltd.;Hu Guoyi;Wu Jianhua;Min Xuefeng;Zhou Guoping CN103992330, 2016, B Location in patent:Paragraph 5-7

94253-98-2
0 suppliers
inquiry

2754-41-8
204 suppliers
$5.00/1g
92298-55-0
4 suppliers
inquiry

2754-41-8
204 suppliers
$5.00/1g
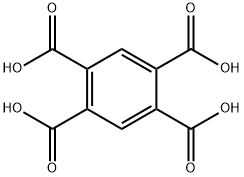
89-05-4
284 suppliers
$5.00/25g

15383-49-0
86 suppliers
$6.00/250mg

2754-41-8
204 suppliers
$5.00/1g

89-05-4
284 suppliers
$5.00/25g

2754-41-8
204 suppliers
$5.00/1g