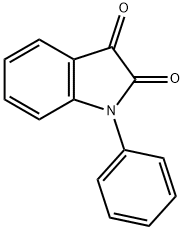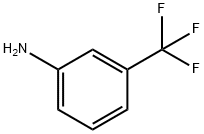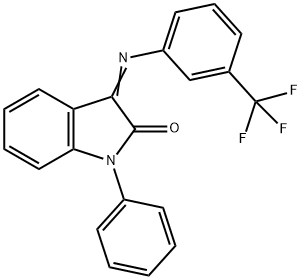
HT-2157 synthesis
- Product Name:HT-2157
- CAS Number:303149-14-6
- Molecular formula:C21H13F3N2O
- Molecular Weight:366.34
Yield: 87%
Reaction Conditions:
in toluene at 95 - 118; for 1 h;Heating / reflux;
Steps:
1 Example 1
To a stirring solution containing toluene (242 mL) and oxalyl chloride (172.8 g, 1.36 mol), under a flow of nitrogen, is added dropwise a solution of diphenylamine (212.64 g, [1.] 26 mol) in toluene (351 [ML).] During the addition, the reaction temperature is kept below [10 °C] with the aid of an ice-bath. After addition is complete, the reaction mixture is heated at [60 °C] for one hour during which time [HC1] gas evolution ceases and a clear light brown solution is obtained. The reaction solution is then distilled [(-175] mm Hg; Pot Temp 72 [°C)] to remove the excess oxalyl chloride. Approximately 300 mL of distillate is collected. The dark red-brown solution is then heated at [120 °C] for approximately 19 h. The reaction solution is cooled to 95 [°C] and a Dean- Stark apparatus is fitted to the flask. A solution of 3- (trifluoromethyl) aniline (263 g, 1.63 mol) dissolved in toluene (529 mL) is then added dropwise over a period of approximately one hour while maintaining the reaction temperature at [95-100 °C.] After addition is complete, the dropping funnel is rinsed with toluene (83 mL) and the liquid is added to the reaction solution. The reaction is heated at [100-118 °C] until the water that is collected is close to the theoretical amount (22.6 mL). The reaction mixture is cooled to [90 °C] and approximately 440 mL solvent is distilled under vacuum (91-95 [°C, ~380] mm Hg). Heptane (650 mL) is then added slowly while maintaining a pot temperature of 90- [95 °C] and the solution is then allowed to cool to room temperature while stirring for overnight. To the resulting thick pasty orange solid, heptane (400 mL) is added, the mixture is stirred vigorously and the solid is collected by filtration. The solid is placed in a vacuum oven to dry at [40 °C] (401.1 g, 87 [%] yield). Compound I may be recrystallized by combining 3 mL toluene per gram of Compound I. The mixture is heated to [60 °C-70 °C] until most of the solids dissolve. The solution is hot filtered and allowed to cool to room temperature, while stirring, for overnight. The orange solids are collected by vacuum filtration and dried in a vacuum oven at 40 [°C] [(80%] recovery yield). Analysis by DSC and XRPD shows that the compound is pure Form I.
References:
SYNAPTIC PHARMACEUTICAL CORPORATION WO2004/14855, 2004, A1 Location in patent:Page 35; 38-39