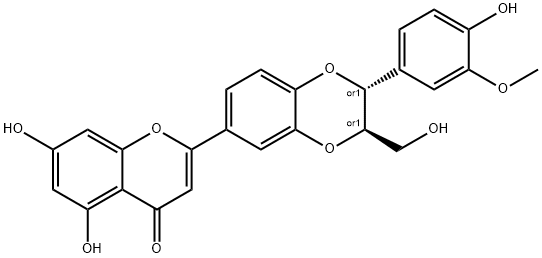
hydnocarpin synthesis
- Product Name:hydnocarpin
- CAS Number:51419-48-8
- Molecular formula:C25H20O9
- Molecular Weight:464.42
802918-57-6
51 suppliers
$8.00/1000mg

51419-48-8
67 suppliers
inquiry
Yield:51419-48-8 55.6%
Reaction Conditions:
Stage #1:silybin with di-isopropyl azodicarboxylate;triphenylphosphine;4-nitro-benzoic acid in tetrahydrofuran at 60; for 1 h;Inert atmosphere;
Stage #2: with sodium hydroxide in tetrahydrofuran at 20; for 1 h;Inert atmosphere;
Steps:
One-pot preparation
Silibinin (482 mg, 1 mmol) was dissolved in 100 mL of dried THF. To the solution Ph3P (1.57 g, 6.0 mmol) and p-nitrobenzoic acid (501 mg, 3.0 mmol) was added, then a solution of diisopropyl azodicarboxylate (808 mg, 4.0 mmol) in dried THF (50 mL) at 60 °C was added dropwise. After addition, the mixture was stirred at 60 °C for 1 h, then evaporated in vacuo until 20 mL of THF was left. To this mixture 2 N NaOH solution (20 mL) was added and stirred at rt for 1hrs until TLC indicated complete hydrolysis. The reaction mixture was acidified with 2 M HCl to pH <4, and extracted with CH2Cl2 (3 × 30 mL). The combined organic phases were washed with brine, dried over anhydrous Na2SO4, and solvent evaporated. The residue was purified by column chromatography (CH2Cl2/MeOH/NH4OH 20/1/0.1) to give title compound 2 as a yellow solid (258 mg, 55.6%). ESI-MS: 465.10 (M+H); 463.12 (M -H). HPLC purity: 100%, tR= 9.29 min (GM1), tR= 17.01 min (GM2). 1H NMR (400 MHz, DMSO-d6) δ 12.90 (s, 1H, 5-OH), 10.84 (s, 1H, 7-OH), 9.17 (s, 1H, 20-OH), 7.67 (d, J = 2.2 Hz, 1H, 13-H), 7.63 (dd, J = 8.5, 2.2 Hz, 1H, 15-H), 7.12 (d, J = 8.5 Hz, 1H, 16-H), 7.05 (d, J = 1.9 Hz, 1H, 18-H), 6.89 (dd, J = 8.2, 1.9 Hz, 1H, 22-H), 6.87 (s, 1H, 3-H), 6.82 (d, J = 8.1 Hz, 1H, 21-H), 6.50 (d, J = 2.1 Hz, 1H, 8-H), 6.19 (d, J = 2.1 Hz, 1H, 6-H), 4.99 (s, 1H, 23-OH), 4.96 (d, J = 7.9 Hz, 1H, 11-H), 4.35 - 4.28 (m, 1H, 10-H), 3.79 (s, 3H, 19-OCH3), 3.61 - 3.53 (m, 23a-H), 3.41 - 3.43 (m, 1H, 23b-H). 1H NMR (400 MHz, CD3OD) δ 7.55 (d, J = 1.9 Hz, 1H, 13-H), 7.53 (dd, J = 8.4, 2.2 Hz, 1H, 15-H), 7.11 (d, J = 8.6 Hz, 1H, 16-H), 7.05 (d, J = 1.9 Hz, 1H, 18-H), 6.94 (dd, J = 8.2, 1.9 Hz, 1H, 22-H), 6.86 (d, J = 8.1 Hz, 1H, 21-H), 6.62 (s, 1H, 3-H), 6.45 (d, J = 2.1 Hz, 1H, 8-H), 6.21 (d, J = 2.1 Hz, 1H, 6-H), 4.97 (d, J = 8.1 Hz, 1H, 11-H), 4.17 (ddd, J = 8.1, 4.3, 2.5 Hz, 1H, 10-H), 3.89 (s, 3H, 19-OCH3), 3.75 (dd, J = 12.5, 2.5 Hz, 1H, 23a-H), 3.51 (dd, J = 12.5, 4.3 Hz, 1H, 23b-H). 13C NMR (101 MHz, DMSO-d6) δ 181.76 (4-CO), 164.28 (2-C), 162.91 (7-C), 161.41 (5-C), 157.33 (9-C), 147.66 (19-C), 147.15 (20-C), 146.86 (12a-C), 143.97 (16a-C), 127.03 (17-C), 123.43 (14-C), 120.66 (22-CH), 120.12 (15-CH), 117.35 (16-CH), 115.34 (21-CH), 115.03 (13-CH), 111.79 (18-CH), 103.85 (3-CH), 103.77 (4a-C), 98.91 (6-CH), 94.07 (8-CH), 78.56 (10-CH), 75.91 (11-CH), 60.03 (23-CH2O), 55.72 (19-OCH3). NMR data are consistent with that of reference.
References:
Huang, Guozheng;Schramm, Simon;Heilmann, Jörg;Biedermann, David;Křen, Vladimír;Decker, Michael [Beilstein Journal of Organic Chemistry,2016,vol. 12,p. 662 - 669] Location in patent:supporting information