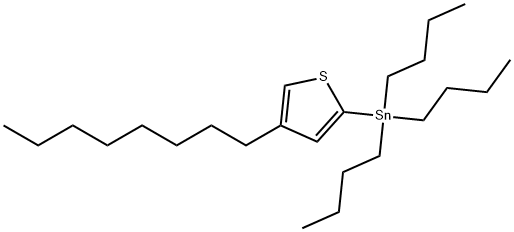
IN1581, Tributyl(4-octylthiophen-2-yl)stannane synthesis
- Product Name:IN1581, Tributyl(4-octylthiophen-2-yl)stannane
- CAS Number:504441-11-6
- Molecular formula:C24H46SSn
- Molecular Weight:485.4
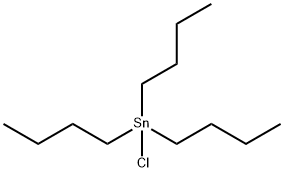
1461-22-9
202 suppliers
$10.00/10g
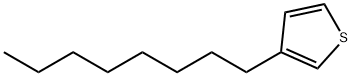
65016-62-8
201 suppliers
$6.00/1g

504441-11-6
17 suppliers
inquiry
Yield:504441-11-6 95%
Reaction Conditions:
Stage #1: 3-octylthiophenewith n-butyllithium in tetrahydrofuran;hexane at -78 - 20; for 2 h;
Stage #2: tributyltin chloride in tetrahydrofuran;hexane at 20;
Steps:
1.1
(1) 3-octylthiophene (5.0 g, 25.5 mmol) was added to 80 mL of anhydrous THF, cooled to -78 ° C, and n-butyllithium (2.4 M in hexanes, 11.7 ml, 28.0 mmol) was slowly added dropwise. The mixture was stirred at -78 ° C for 1 hour and allowed to react at room temperature for 1 hour. The reaction system was cooled to -78 ° C. 2M trimethyltin chloride (9.1 g, 28.0 mmol) in THF was added to the reaction system through a syringe for 1 hour and the reaction was allowed to warm to room temperature overnight. The reaction was quenched by adding 20 mL of KF aqueous solution to the reaction, and the mixture was extracted twice with anhydrous ether. The organic phases were combined, dried over anhydrous sodium sulfate, filtered and evaporated to dryness to give yellow liquid product 1 (11.7 g, 95%).
References:
CN106397428,2017,A Location in patent:Paragraph 0044; 0052
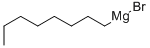
17049-49-9
100 suppliers
$75.00/25g

504441-11-6
17 suppliers
inquiry