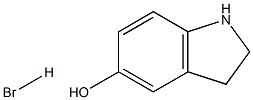
Indolin-5-ol hydrobroMide synthesis
- Product Name:Indolin-5-ol hydrobroMide
- CAS Number:1221257-43-7
- Molecular formula:C8H10BrNO
- Molecular Weight:216.0751
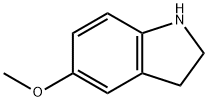
21857-45-4
124 suppliers
$15.00/100mg

1221257-43-7
8 suppliers
inquiry
Yield:1221257-43-7 60%
Reaction Conditions:
with hydrogen bromide;acetic acid in water;Reflux;
Steps:
8.B
A solution of the product Step A (0.32 g; 2.14 mmol) in 48% HBr/ AcOH 1 :1 (10 ml) was refluxed overnight, then evaporated to dryness under reduced pressure. The residue was dissolved in EtOH (10 ml) and the solvent was removed under reduced pressure. This process was repeated three times and the residue was suspended in acetonitrile. The greyish solid formed was filtered off, washed with fresh CH3CN and dried to give a hydrobromide salt of the title compound (0.28 g; 60%). . 1 H-NMR (D2O ) 3.18 (tr, 2H, J = 7.8 Hz); 3.78 (tr, 2H, J = 7.8 Hz); 4.66 (HDO); 6.78 (d, 1 H, J = 8.6 Hz); 6.87 (s, 1 H); 7.23 (d, 1 H, J = 8.6 Hz).
References:
WO2010/42998,2010,A1 Location in patent:Page/Page column 54