Ingenol synthesis
- Product Name:Ingenol
- CAS Number:30220-46-3
- Molecular formula:C20H28O5
- Molecular Weight:348.43
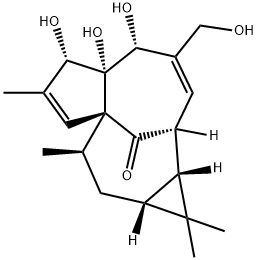
54706-99-9
112 suppliers
inquiry

30220-46-3
233 suppliers
$27.00/500μg
Yield:30220-46-3 70%
Reaction Conditions:
with selenium(IV) oxide in 1,4-dioxane;formic acid at 80; for 2 h;
Steps:
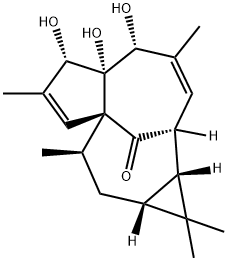
20 Example 20: Allylic Oxidation of 20
To a solution of 20 (4.9 mg, 0.015 mmol, 1.0 equiv) in dioxane (500 μΕ) and formic acid (250 μΕ) was added Se02 (17 mg, 0.15 mmol, 10.0 equiv). The suspension was heated to 80 °C for 2 h then cooled and quenched by the addition of saturated aqueous NaHC03 (4 mL) and Et20 (4 mL). The aqueous layer was removed and 10% sodium hydroxide (4 mL) was added. The biphasic mixture was vigorously shaken and the organic layer was removed. The aqueous layer was extracted with Et20 (2 x 4 mL) and the combined organic layers were dried and evaporated. The crude product was purified by column chromatography (1: 1 DCM:EtOAc) to give ingenol (21, 3.6 mg, 70%) as a white film (for NMR data describing ingenol (21), see Appendio et al., "An Expeditious Procedure for the Isolation of Ingenol from the Seeds of Euphorbia lathyris," J. Nat. Prod., 62:76-79, 1999, incorporated herein by reference in its entirety for all purposes).
References:
LEO LABORATORIES LIMITED;BARAN, Phillipe S.;JØRGENSEN, Lars;KUTTRUFF, Christian A.;MCKERRALL, Steven J.;YEH, Chien-Hung WO2014/191457, 2014, A1 Location in patent:Page/Page column 84
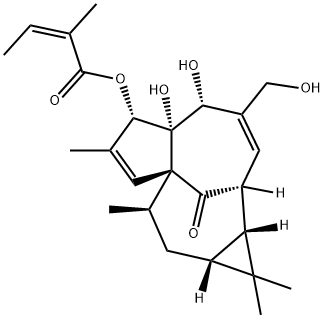
75567-37-2
161 suppliers
$65.00/500μg

30220-46-3
233 suppliers
$27.00/500μg
![1H-2,8a-Methanocyclopenta[a]cyclopropa[e]cyclodecen-11-one, 4-[(acetyloxy)methyl]-1a,2,5,5a,6,9,10,10a-octahydro-5,5a,6-trihydroxy-1,1,7,9-tetramethyl-, (1aR,2S,5R,5aR,6S,8aS,9R,10aR)-](/CAS/20210111/GIF/64280-37-1.gif)
64280-37-1
0 suppliers
inquiry

30220-46-3
233 suppliers
$27.00/500μg