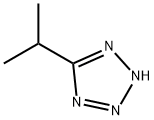
Isopropyl Tetrazole synthesis
- Product Name:Isopropyl Tetrazole
- CAS Number:6280-28-0
- Molecular formula:C4H8N4
- Molecular Weight:112.13
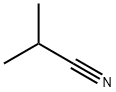
78-82-0
0 suppliers
$14.14/5gm:

6280-28-0
15 suppliers
$84.00/250mg
Yield:6280-28-0 43%
Reaction Conditions:
with sodium azide;cerium(III) chloride heptahydrate in water;isopropyl alcohol at 160; for 1 h;Microwave irradiation;
Steps:
General Methodology for the Synthesis of 5-Substituted 1H-Tetrazoles
General procedure: Synthesis of 5-(thiophen-3-yl)-1H-tetrazole (2a). 3-Thiophenecarbonitrile 1a (218 mg, 2 mmol), NaN3 (260 mg,4 mmol), CeCl3·7H2O (75 mg, 0.2 mmol), and 8 mL of a 3:1isopropanol/water mixture were added to a 30-mL Pyrexmicrowave vessel and capped. The microwave vessel wasthen placed in a multi-mode microwave reactor. The reactionwas magnetically stirred and heated for 1 hour at 160 °C.The pressure in the vessel was not determined. The reactionwas monitored by TLC using an ether/hexane mixture (typically50/50) for development. After cooling, the reactionmixture was diluted with saturated aqueous sodium bicarbonate(20 mL) and washed with ethyl acetate (2 x 15 mL).The aqueous sodium bicarbonate layer was cooled with iceand acidified to a pH of 2 or less with concentrated hydrochloricacid, which was added drop-wise. The precipitateformed was extracted with ethyl acetate (3 x 15 mL). Thecombined organic layers were dried over anhydrous sodiumsulfate and decanted into a tared round bottom flask. Theorganic layer was concentrated under reduced pressure. Thetetrazole product was recrystallized from ethyl acetate andhexane. All reagents mentioned above were used unpurified.
References:
Dudley, Joshua;Feinn, Liana;Defrancesco, Heather;Lindsay, Erica;Coca, Adiel;Roberts, Elizabeth Lewis [Medicinal Chemistry,2018,vol. 14,# 6,p. 550 - 555]