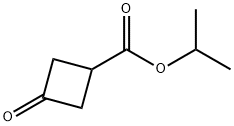
isopropyl3-oxocyclobutane-1-carboxylate synthesis
- Product Name:isopropyl3-oxocyclobutane-1-carboxylate
- CAS Number:130111-95-4
- Molecular formula:C8H12O3
- Molecular Weight:156.18
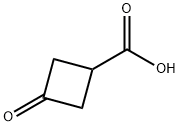
23761-23-1
450 suppliers
$6.00/1g
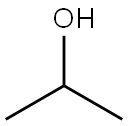
67-63-0
1549 suppliers
$16.00/25ML

130111-95-4
22 suppliers
inquiry
Yield:-
Reaction Conditions:
with toluene-4-sulfonic acid at 80; for 19 h;
Steps:
2 (Example 2)Alternative Preparation of N-((1S, 3S) -3- (Methyl (7H-pyrrolo [2,3-d] pyrimidin-4-yl) amino) cyclobutyl) propane-1-sulfonamide Isopropyl 3-oxocyclobutane-1 -Carboxylate (A)
Commercially available3-cyclobutanone carboxylic acid(175 g)Dissolve in 2-propanol (1050 mL)p-Toluenesulfonic acid monohydrate was added(11.85 g, 4 mol%).The solution was heated to 80 ° C. and stirred for 19 hours.The reaction was judged complete by UPLCMS and was cooled.The reaction was concentrated to a light yellow oil and diluted with 1000 mL of MTBE.The solution was washed with 300 mL of saturated sodium bicarbonate and separated.The aqueous layer was discarded and washed with an additional 200 mL of saturated sodium bicarbonate.The layers were separated and the aqueous layer was discarded.The MTBE layer was dried with 200 mL of brine and then magnesium sulfate.The MTBE solution was then concentrated to a pale yellow oil.
References:
Pfizer Inc;Arora, Kapildev Kashmirilal;Deforest, Jacob Cole;Hills, Andrew Kevern;Jones, Brian Patrick;Jones, Kris Nicola;Lewis, Chad Arthur;Rane, Anil Mahadeo JP2020/7308, 2020, A Location in patent:Paragraph 0095