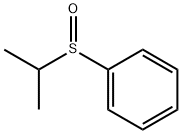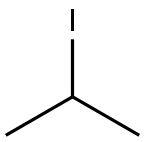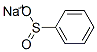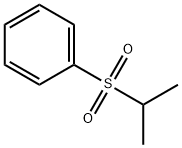
ISOPROPYLPHENYL SULFONE synthesis
- Product Name:ISOPROPYLPHENYL SULFONE
- CAS Number:4238-09-9
- Molecular formula:C9H12O2S
- Molecular Weight:184.26
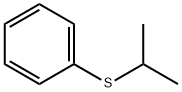
3019-20-3
37 suppliers
$18.00/50g

4238-09-9
10 suppliers
$496.49/5MG
Yield:4238-09-9 100%
Reaction Conditions:
with oxygen in lithium hydroxide monohydrate at 20; under 15001.5 Torr; for 0.333333 h;Sonication;Green chemistry;
Steps:
2.5. General procedure for oxidation of sulfides
General procedure: Reactions were performed at r.t. and in the open air inside a25 mL flask equipped with a magnetic stirrer bar or under US irradiation(100 w). A mixture of sulfides (1 mmol) and DFNSILMo368(0.79 mg) was stirred under the open air at r.t. for therequired time. The reaction procedure was observed by TLC andproduct efficiencies were specified through GC analysis. The productswere purified by chromatography over silica gel (Eluent: ethylacetate/n-hexane 40:60 for sulfoxides isolation and 15:85 for sulfonesisolation). The products were specified using their IR andNMR spectra or compared with the retention time of authenticGC samples.
References:
Xiao, BoAn;Sadeghzadeh, Seyed Mohsen [Journal of Molecular Liquids,2022,vol. 363,art. no. 119921]
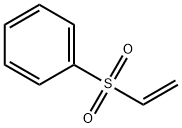
5535-48-8
262 suppliers
$12.00/1g
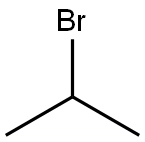
75-26-3
469 suppliers
$10.00/10g

4238-09-9
10 suppliers
$496.49/5MG
![Benzene, 1,1'-[(1-methylethylidene)bis(sulfonyl)]bis-](/CAS/20210305/GIF/39863-09-7.gif)
39863-09-7
0 suppliers
inquiry

4238-09-9
10 suppliers
$496.49/5MG