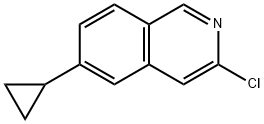
Isoquinoline, 3-chloro-6-cyclopropyl- synthesis
- Product Name:Isoquinoline, 3-chloro-6-cyclopropyl-
- CAS Number:1578483-81-4
- Molecular formula:C12H10ClN
- Molecular Weight:203.67
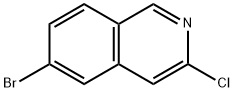
552331-06-3
91 suppliers
$43.00/100mg
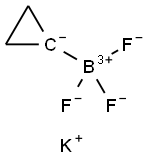
1065010-87-8
152 suppliers
$6.00/250mg

1578483-81-4
0 suppliers
inquiry
Yield:1578483-81-4 41%
Reaction Conditions:
with palladium diacetate;caesium carbonate;catacxium A in water;toluene at 100; for 2 h;Microwave irradiation;
Steps:
2; D Preparation 2: 3-Chloro-6-cyclopropylisoquinoline Method D
Preparation 2: 3-Chloro-6-cyclopropylisoquinoline Method D A suspension of 6-bromo-3-chloroisoquinoline (50 mg, 0.21 mmol), potassium cyclopropyltrifluoroborate (40 mg, 0.27 mmol), Pd(OAc)2 (3.7 mg, 0.016 mmol), Cs2C03 (202 mg, 0.62 mmol) and di(1 -adamantyl)-n-butylphosphine (5.54 mg, 0.015 mmol) in toluene/water (4/0.4 mL) was stirred at 100 under microwave irradiation for 120 minutes. The reaction mixture was filtered, concentrated in vacuo and purified using Biotage silica gel column chromatography eluting with DCM to afford the title compound (17 mg, 41 %). 1 H NMR (500 MHz, CDCI3): δ 8.97 (s, 1 H), 7.85 (d, J = 8.6 Hz, 1 H), 7.61 (d, J = 1.0 Hz, 1 H), 7.46 - 7.38 (m, 1 H), 7.33 - 723 (m, 1 H), 2.05 - 2.01 (m, 1 H), 1 .19 - 1 .10 (m, 2H), 0.91 - 0.85 (m, 2H). LCMS (ESI) Rt = 2.77 minutes MS m/z 204 [M+H]+
References:
WO2014/37751,2014,A1 Location in patent:Paragraph 00254-00256