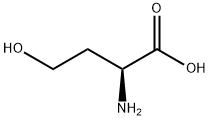
L-Homoserine synthesis
- Product Name:L-Homoserine
- CAS Number:672-15-1
- Molecular formula:C4H9NO3
- Molecular Weight:119.12
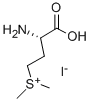
3493-11-6
34 suppliers
$119.00/500mg

672-15-1
392 suppliers
$5.00/100mg
Yield:672-15-1 92%
Reaction Conditions:
with water;sodium hydrogencarbonate pH=2 - 5;Reflux;
Steps:
3
(2S)-Methionine methylsulfonium iodide (9.4 g, 32.3 mmol) was dissolved in water (30 mL) in a three-necked round-bottom flask and heated to reflux. Sodium bicarbonate (2.71 g, 32.3 mmol) was dissolved in water (40 mL), placed in a dropping funnel and added dropwise to the reaction mixture until the pH of the solution rose to 5.0 (monitored using a VWR symphony SB20 pH meter). The reaction was allowed to proceed until the pH dropped to 2.0 (~ 15 min), whereupon base was added as previously described (addition over 6 h). The reaction mixture was then allowed to stir with heating at reflux for 12 h before being cooled, transferred to a 50 mL round-bottom flask and concentrated to give a clear oil. The oil was dissolved in water (5 mL) and ethanol (10 mL), and treated with acetone (150 mL) to produce a white precipitate that was collected by filtration. The precipitate was dissolved in water (20 mL) and freeze-dried, giving (S)-2-amino-4-hydroxybutyric acid (2S)-9 as a white solid (3.55 g, 92%). Rf = 0.73 (40% EtOH / H2O); mp: 175- 177 0C (EtOH / Et2O) (Lit."a mp: 182-183 0C); [α]D20 -8.5 (c 1.0, H2O) [Lit."a [α]D20 -8.9 (c 2.0, H2O)]; 1H NMR (300 MHz, D2O) δ 1.86-1 .93 (m, 1H, β- CAVH), 1.99-2.05 (m, 1H, β-CHAV), 3.63-3.67 (m, 2H, γ-CH2) and 3.71 (dd, 1 H, J 7.5, 4.8, α-CH); 13C NMR (75 MHz, D2O) δ 32.9 (CH2), 54.1 (CH), 59.4 (CH2), 175.2 (C=O); m/z (ESI) 120 (MH+, 100%) and 102 (1 ).
References:
WO2010/105367,2010,A1 Location in patent:Page/Page column 27
![TERT-BUTYL N-[1-(FURAN-2-YL)-3-HYDROXYPROPYL]CARBAMATE](/CAS/GIF/1149755-80-5.gif)
1149755-80-5
2 suppliers
inquiry

672-15-1
392 suppliers
$5.00/100mg
29625-73-8
4 suppliers
inquiry

672-15-1
392 suppliers
$5.00/100mg
![β-D-Glucopyranosylamine, 2-(acetylamino)-4-O-[2-(acetylamino)-2-deoxy-β-D-glucopyranosyl]-2-deoxy-](/CAS/20210305/GIF/102039-77-0.gif)
102039-77-0
0 suppliers
inquiry
![D-Glucitol, 1-(acetylamino)-4-O-[2-(acetylamino)-2-deoxy-β-D-glucopyranosyl]-2-amino-1,2-dideoxy- (9CI)](/CAS/20210305/GIF/118943-00-3.gif)
118943-00-3
0 suppliers
inquiry
![D-Glucitol, 2-(acetylamino)-4-O-[2-(acetylamino)-2-deoxy-β-D-glucopyranosyl]-1-amino-1,2-dideoxy-](/CAS/20210305/GIF/119009-35-7.gif)
119009-35-7
0 suppliers
inquiry

29625-73-8
4 suppliers
inquiry

672-15-1
392 suppliers
$5.00/100mg
![β-D-Glucopyranosylamine, 2-(acetylamino)-4-O-[2-(acetylamino)-2-deoxy-β-D-glucopyranosyl]-2-deoxy-](/CAS/20210305/GIF/102039-77-0.gif)
102039-77-0
0 suppliers
inquiry
![D-Glucitol, 2-(acetylamino)-4-O-[2-(acetylamino)-2-deoxy-β-D-glucopyranosyl]-1-amino-1,2-dideoxy-](/CAS/20210305/GIF/119009-35-7.gif)
119009-35-7
0 suppliers
inquiry
29886-32-6
3 suppliers
inquiry
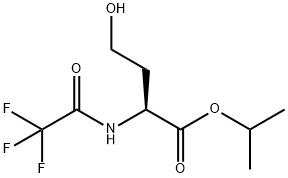
159487-41-9
0 suppliers
inquiry

672-15-1
392 suppliers
$5.00/100mg