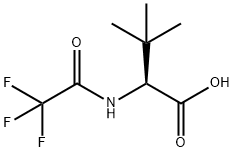
L-Valine, 3-methyl-N-(trifluoroacetyl)- (9CI) synthesis
- Product Name:L-Valine, 3-methyl-N-(trifluoroacetyl)- (9CI)
- CAS Number:666832-71-9
- Molecular formula:C8H12F3NO3
- Molecular Weight:227.18
20859-02-3
622 suppliers
$9.00/5G
383-63-1
503 suppliers
$10.00/25g

666832-71-9
105 suppliers
inquiry
Yield:666832-71-9 86%
Reaction Conditions:
with sodium methoxide in methanol at 40; for 2.5 h;
Steps:
98.1 Step Synthesis of 3-methyl-N-(trifluoroacetyl)-L-valine (C91).
A solution of sodium methoxide in methanol (25 weight%; 28.5 mL, 124 mmol) was added to a solution of 3-methyl-L-valine (99%, 15 g, 113 mmol) in methanol (30 mL). Ethyl trifluoroacetate (130 mmol) was then added and the reaction mixture was stirred at 40 °C until the reaction was complete (approximately 2.5 hours), whereupon it was cooled to 20 °C. After addition of hydrochloric acid (1 M; 136 ml, 136 mmol), the mixture was diluted with ethyl acetate (150 mL) and the layers were separated. The organic layer was washed twice with saturated aqueous sodium chloride solution, dried over magnesium sulfate, and filtered. Heptane was added to the filtrate, whereupon the solution was concentrated at 50 °C to a volume of 5 mL/g. This procedure was carried out twice; after the second distillation, seed crystals of C91 (50 mg; see below) were added. The resulting solid was collected via filtration, washed with heptane, and dried at 40 °C to provide C91 as an off-white solid. Yield: 22.2 g, 97.7 mmol, 86%. The seed crystals used above were obtained from a similar reaction carried out using 3-methyl-L-valine; after the organic layer containing C91 had been dried over magnesium sulfate and filtered, concentration in vacuo provided a solid. A portion of this solid was used as the seed material. Physicochemical data was obtained on samples of C91 obtained from reactions carried out in the same manner. HRMS-ESI+ ( m/z) [M+H]+ Calculated for C8H13F3NO3, 228.0842; Found, 228.0842. Primary ion observed as C8H11F3NNa203 [M+Na+]: Calculated, 272.0481 ; Found, 272.0482. 1H NMR (600 MHz, DMSO-d6) δ 13.05 (s, 1H), 9.48 (d, J = 8.9 Hz, 1H), 4.21 (d, J = 8.9 Hz, 1H), 1.00 (s, 9H). 13C NMR (150.8 MHz, DMSO-d6) δ 170.9, 156.6 (q, 2JCF = 36.9 Hz), 115.8 (q, 1JCF = 287.7 Hz), 61.0, 33.6, 26.5.
References:
WO2021/250648,2021,A1 Location in patent:Page/Page column 263; 267-268