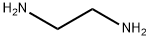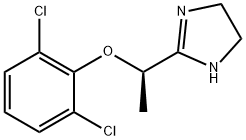
Levlofexidine synthesis
- Product Name:Levlofexidine
- CAS Number:81447-78-1
- Molecular formula:C11H12Cl2N2O
- Molecular Weight:259.13
Yield:81447-78-1 85%
Reaction Conditions:
Stage #1: (R)-(-)-2-(2,6-dichlorophenoxy)propionamidewith trimethoxonium tetrafluoroborate in dichloromethane; for 36 h;
Stage #2: ethylenediamine in dichloromethane at 0 - 20;
Steps:
(-)-2-[1-(2,6-dichlorophenoxy)-ethyl]-1,3-diazacyclopent-2-ene (3). A mixture of 25 (25 g, 106.8 mmol), trimethyloxonium tetrafluoroborate (16.6 g, 110 mmol) and CH2Cl2 (600 mL) was stirred for 36 hours, during which the coarse suspension changed to a clear, colorless solution. Cooling of this solution of 0° C. was followed by a dropwise addition of ethylene diamine (7.00 g, 1.1 equiv) over 10 minutes. The resulting solution was warmed to room temperature, diluted with absolute ethanol (200 mL) and then evaporated to dryness. The pasty white residue was partitioned between 5% aqueous K2CO3 (200 mL) and CH2Cl2 (500 mL). The organic layer was dried (Na2SO4) and evaporated under reduced pressure. Recrystallization of the residue from boiling hexanes (500 mL) afforded pure 3 as white needles that were removed by filtration and dried in air (23.52 g, 85%). 1H NMR (300 MHz, d6-DMSO) δ ppm 7.46-7.44 (m, 2H, Ar), 7.14 (t, J=7.8 Hz, 1H, Ar), 6.45 (s, 1H, NH), 4.79 (q, J=6.6 Hz, 1H), 3.43-3.37 (s, br, 4H), 1.47 (d, J=6.6 Hz, 3H). 13C NMR (75 MHz, d6-DMSO) δ ppm 166.3, 150.0, 130.0, 129.6, 129.5, 129.2, 126.6, 77.3, 50.4 (very broad), 19.2; mp=129-130° C. (hexanes); [α]23D=-80.2° (c 1.0, EtOH) (lit.4 [α]20D=-76.4° (c 1.0, EtOH).
References:
US2010/87657,2010,A1 Location in patent:Page/Page column 6
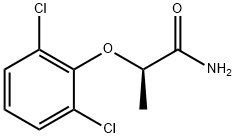
87789-42-2
3 suppliers
inquiry

81447-78-1
16 suppliers
inquiry
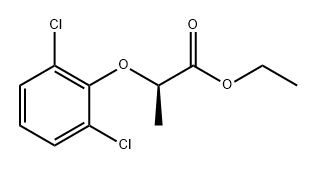
383898-16-6
0 suppliers
inquiry

81447-78-1
16 suppliers
inquiry
101934-13-8
0 suppliers
inquiry

81447-78-1
16 suppliers
inquiry