LGD-4033 synthesis
- Product Name:LGD-4033
- CAS Number:1165910-22-4
- Molecular formula:C14H12F6N2O
- Molecular Weight:338.25
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
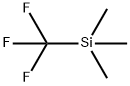
81290-20-2
417 suppliers
$13.00/1g
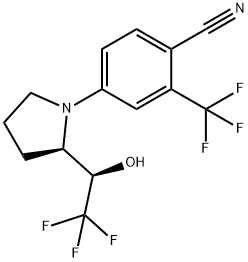
![4-[(2R)-2-[(1S)-2,2,2-Trifluoro-1-hydroxyethyl]-1-pyrrolidinyl]-2-(trifluoromethyl)-benzonitrile](/CAS/20200401/GIF/CB85144550.gif)
1165910-23-5
6 suppliers
inquiry

1165910-22-4
183 suppliers
$28.00/5mg
Yield:-
Steps:
1
A mixture of D-prolinol, 4-fluoro-2-trifluoromethylbenzonitrile, and triethylamine in THF was stirred over night at 60°C. Standard work-up of the reaction mixture provided R-4-(2-hydroxylmethylpyrrolidinyl)-2-trifluoromethyl- benzonitrile in moderate yield. The intermediate alcohol was oxidized by sulfur trioxide pyridine complex to give /?-4-(2-formyl-pyrrolidinyl)-2-trifluoromethyl- benzonitrile. The aldehyde intermediate was treated with trimethyl(trifluoromethyl)- silane to provide a mixture of two diastereomers. HPLC separation generated pure forms of Compounds 101 and 102. Compound 101: 1H-NMR (500 MHz, CDCl3) 7.59 (d, J= 8.8, IH), 7.07 (d, J= 2.9, IH), 6.92 (dd, J= 8.8 and 2.9, IH), 4.24 (t, J= 7.5, IH), 3.91 (t, J= 6.3, IH), 3.63 (dd, J= 7.8 and 9.3, IH), 3.29 (td, J= 6.9 and 9.7, IH), 2.55 (s, IH), and 2.05- 2.23 (m, 4H).Compound 102: 1H-NMR (500 MHz, acetone-d6) 7.78 (d, J= 8.8, IH), 7.01 (d, J= 2.9, IH), 6.94 (dd, J= 8.8 and 2.9, IH), 5.68 (bd, J= 3.3, IH), 4.44-4.50 (m, IH), 4.36 (d, J= 8.3, IH), 3.64-3.77 (m, IH), 3.44 (td, J= 9.8 and 7.8, IH), 2.30-2.47 (m, 2H), and 2.07-2.17 (m, 2H).
References:
LIGAND PHARMACEUTICALS INCORPORATED WO2009/82437, 2009, A2 Location in patent:Page/Page column 145