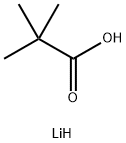
lithium pivalate synthesis
- Product Name:lithium pivalate
- CAS Number:14271-99-9
- Molecular formula:C5H11LiO2
- Molecular Weight:110.08
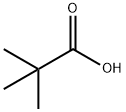
75-98-9
437 suppliers
$10.00/10g

14271-99-9
3 suppliers
inquiry
Yield:14271-99-9 100%
Reaction Conditions:
with methyllithium in tetrahydrofuran;diethyl ether at 0; for 0.75 h;Inert atmosphere;
Steps:
4A
Pivalic acid (5.11 g, 5.74 mL, 50.0 mmol) was placed in a dry and argon-flushed 250 mL Schlenk-iiask, equipped with a magnetic stirring bar and a septum, and dissolved in dry THF (30 mL). The solution was cooled to 0 °C and methyllithium (32.4 mL, 1.70 M in diethyl ether, 55.0 mmol) was added dropwise over a period of 45 min. The solvent was removed in vacuo and LiOPiv was obtained as a slightly yellow solid in quantitative yield.Zinc powder (490 mg, 7.5 mmol) and LiCl (318 mg, 7.5 mmol) were placed in a Schlenk- flask, equipped with a magnetic stirrer and a septum, dried for 5 min at 400 °C (heat gun) in high vacuum and then dissolved in 7.0 mL of dry THF. 4 drops of 1,2-dibromoethane were added and the mixture was heated to boiling for the activation of the zinc dust. After cooling to 22 °C 3-iodopropionitrile (905 mg, 5.0 mmol) was added dropwise and the mixture was stirred for 2 h at 22 °C. The stirring of the reaction mixture was stopped and the excess zinc dust was sedimented. The supernatant solution was transferred to another Schlenk-iiask containing a solution of lithium pivalate (811 mg, 1.50 mmol) prepared as described above in 5.0 mL of dry THF. The mixture was stirred for 15 min at 22 °C and then the solvent was removed in vacuo. (2-cyanoethyl)zinc pivalate was obtained as a yellow solid (2.94 g). The content of active zinc species was determined by titration of 398 mg of the reagent with a stock solution of iodine (1.0 M in THF). A concentration of 1020 mg / mmol was determined which corresponds to a yield of 58 %.
References:
WO2012/85168,2012,A1 Location in patent:Page/Page column 27