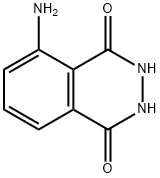
Luminol synthesis
- Product Name:Luminol
- CAS Number:521-31-3
- Molecular formula:C8H7N3O2
- Molecular Weight:177.16
3682-15-3
159 suppliers
$74.00/5g

521-31-3
547 suppliers
$5.00/100mg
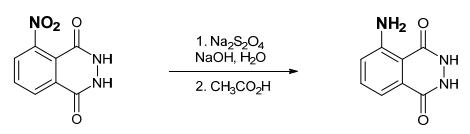
Yield:521-31-3 83%
Reaction Conditions:
with formic acid;10% Pd/C;hydrogen at 35; for 5 h;Reagent/catalyst;
Steps:
3.3
To a three-necked flask was added 5 gl0 wt% palladium content meter) palladium on carbon, Stir evenly after heating to 35 ° C, A solution of 260 g of 85 wt% aqueous solution of formic acid was slowly added dropwise with stirring, The dropping time was 2 hours, and after the dropwise addition, Continue to react for 3 hours, the point plate to confirm 3-nitro phthalic acid hydrazide has been completely consumed, The pH was adjusted to 3-4 with 35 wt% concentrated hydrochloric acid and heated to 100 ° C, Hot filter, the filtrate cooled to l ° C, a large number of precipitation, Precipitate out completely after filtration, the filtrate for organic solvent recovery, The filter cake was washed with 300 ml of deionized water and dried to give 182 g of luminol (3-aminophthalohydrazide).
References:
CN106810501,2017,A Location in patent:Paragraph 0062
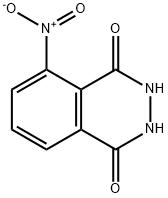
2518-24-3
154 suppliers
$50.00/500mg

521-31-3
547 suppliers
$5.00/100mg
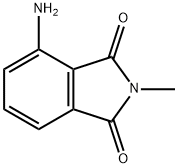
2257-85-4
63 suppliers
$60.00/500mg

521-31-3
547 suppliers
$5.00/100mg
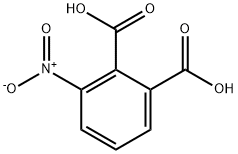
603-11-2
639 suppliers
$15.00/25g

521-31-3
547 suppliers
$5.00/100mg
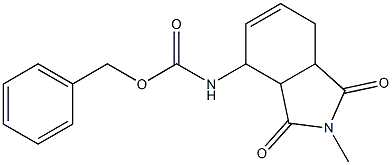
768370-07-6
5 suppliers
inquiry

521-31-3
547 suppliers
$5.00/100mg