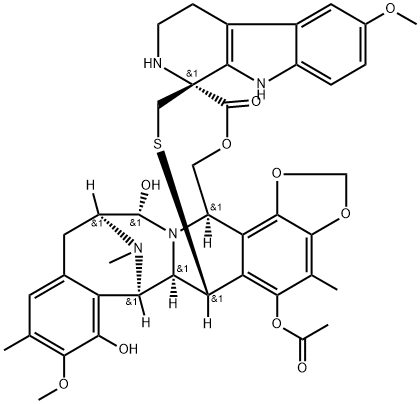
Lurbinectedin synthesis
- Product Name:Lurbinectedin
- CAS Number:497871-47-3
- Molecular formula:C41H44N4O10S
- Molecular Weight:784.88

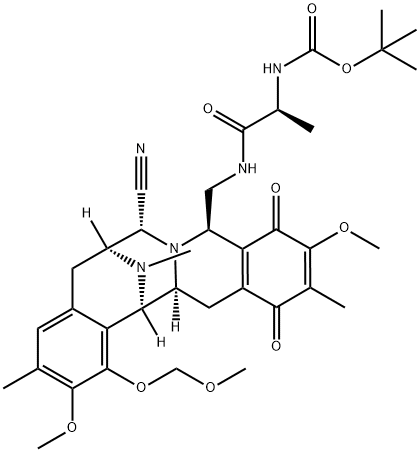
308359-25-3
1 suppliers
inquiry

497871-47-3
103 suppliers
inquiry
Yield:-
Steps:
Multi-step reaction with 16 steps
1.1: water; lithium hydroxide / tetrahydrofuran
2.1: hydrogen / palladium 10% on activated carbon / N,N-dimethyl-formamide / 2 h / 23 °C
2.2: 3 h / 23 °C
3.1: trifluoroacetic acid / dichloromethane / 1.5 h / 23 °C
4.1: dichloromethane / 2 h / 23 °C
5.1: chloro-trimethyl-silane; methanol / 1.5 h / 23 °C
6.1: water; disodium hydrogenphosphate; sodium nitrite; phosphoric acid / dichloromethane / 19 h / 23 °C
7.1: sodium hydride / tetrahydrofuran; mineral oil / 2 h / 0 - 23 °C
8.1: dmap; N-ethyl-N,N-diisopropylamine; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride / dichloromethane / 3 h / 23 °C
9.1: tri-n-butyl-tin hydride; acetic acid / bis-triphenylphosphine-palladium(II) chloride / dichloromethane / 0.75 h / 23 °C
10.1: hydrogen / palladium 10% on activated carbon / acetonitrile / 2.5 h / 23 °C
10.2: 20 h / 23 - 70 °C
11.1: benzeneseleninic anhydride / dichloromethane
12.1: trifluoromethylsulfonic anhydride; N-ethyl-N,N-diisopropylamine / dimethyl sulfoxide; tert-butyl alcohol
13.1: methanesulfonic acid / dichloromethane
14.1: oxalic acid; 1,8-diazabicyclo[5.4.0]undec-7-ene; N-methylpyridine-4-carboxaldehyde iodide
15.1: acetic acid
16.1: water; silver nitrate / acetonitrile
References:
WO2011/147828,2011,A1
![Spiro[6,16-(epithiopropanoxymethano)-7,13-imino-12H-1,3-dioxolo[7,8]isoquino[3,2-b][3]benzazocine-20,1'-[1H]pyrido[3,4-b]indole]-14-carbonitrile, 5-(acetyloxy)-2',3',4',6,6a,7,9',13,14,16-decahydro-8-hydroxy-6',9-dimethoxy-4,10,23-trimethyl-19-oxo-, (1'R,6R,6aR,7R,13S,14R,16R)- (9CI)](/CAS/20211123/GIF/497871-10-0.gif)
497871-10-0
2 suppliers
inquiry

497871-47-3
103 suppliers
inquiry
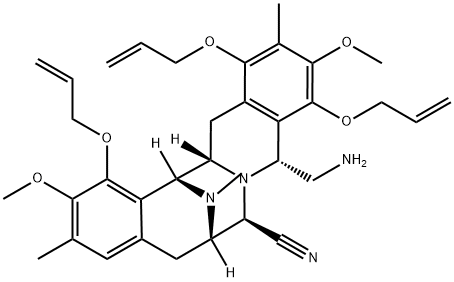
1350888-49-1
3 suppliers
inquiry

497871-47-3
103 suppliers
inquiry

1350888-50-4
3 suppliers
inquiry

497871-47-3
103 suppliers
inquiry

308359-24-2
1 suppliers
inquiry

497871-47-3
103 suppliers
inquiry