LY 2157299 synthesis
- Product Name:LY 2157299
- CAS Number:700874-72-2
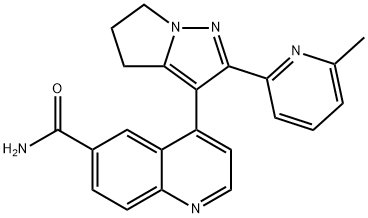
- Molecular formula:C22H19N5O
- Molecular Weight:369.42
![6-Quinolinecarboxylic acid, 4-[5,6-dihydro-2-(6-Methyl-2-pyridinyl)-4H-pyrrolo[1,2-b]pyrazol-3-yl]-, Methyl ester](/CAS/GIF/476475-44-2.gif)
476475-44-2

700874-72-2
Methyl 4-(2-(6-methylpyridin-2-yl)-5,6-dihydro-4H-pyrrolo[1,2-b]pyrazol-3-yl)quinoline-6-carboxylate (raw material) was reacted with 60 mL of 7N ammonia-methanol solution in a stainless steel pressure vessel heated to 90°C for 66 hours. The pressure was raised to about 80 PSI during the reaction and needed to be maintained until the reaction was complete. At the end of the reaction, the vessel was cooled and the brown mixture was concentrated under vacuum. The residual solid was purified by passing through two 12 g Redi-Sep fast columns using acetone as eluent. The fractions containing the target product were collected and combined, followed by vacuum concentration. The resulting near-white solid was suspended in dichloromethane, diluted with the addition of hexane and filtered to afford the off-white solid product 4-[5,6-dihydro-2-(6-methyl-2-pyridinyl)-4H-pyrrolo[1,2-b]pyrazol-3-yl]-6- quinolinecarboxamide in a yield of 1.104 g and 63.8%. Mass spectral analysis showed ES + = 370 (M + 1).
![6-Quinolinecarboxylic acid, 4-[5,6-dihydro-2-(6-Methyl-2-pyridinyl)-4H-pyrrolo[1,2-b]pyrazol-3-yl]-, Methyl ester](/CAS/GIF/476475-44-2.gif)
476475-44-2
8 suppliers
inquiry

700874-72-2
217 suppliers
$22.00/2mg
Yield:700874-72-2 63.8%
Reaction Conditions:
with ammonia in methanol at 90; under 4137.29 Torr; for 66 h;
Steps:
2.G G. Preparation of 2- (6-METHYL-PYRIDIN-2-YL)-3- [6-AMIDO-QUINOLIN-4-YL)-5, 6- DIHYDRO-4H-PYRROLO [1, 2-B] PYRAZOLE
Warm a mixture OF 4- [2- (6-METHYL-PYRIDIN-2-YL)-5, 6-dihydro-4H-pyrrolo [1, 2- B] PYRAZOL-3-YL] -QUINOLINE-6-CARBOXYLIC ACID METHYL ESTER IN 60 ML 7 N AMMONIA IN methanol to 90 °C in a stainless steel pressure vessel for 66 hours. The pressure will rise to about 80 PSI. Maintain the pressure for the duration of the reaction. Cool the vessel and concentrate the brown mixture in vacuo. Purify the residual solid on two 12 g Redi- Pak cartridges coupled in series eluting with acetone. Combine appropriate fractions and concentrate in vacuo. Suspend the resulting nearly white solid in methylene chloride, dilute with hexane, and filter. The collected off-white solid yields 1.104 g (63.8%) of the desired title product. MS ES+ = 370 (M+1).
References:
ELI LILLY AND COMPANY WO2004/48382, 2004, A1 Location in patent:Page 10
![4-(2-(6-methylpyridin-2-yl)-5,6-dihydro-4H-pyrrolo[1,2-b]pyrazol-3-yl)quinoline-6-carbonitrile](/CAS/20180703/GIF/924898-13-5.gif)
924898-13-5
11 suppliers
inquiry

700874-72-2
217 suppliers
$22.00/2mg