
METHYL 1-(4-NITROPHENYL)-4-PIPERIDINECARBOXYLATE synthesis
- Product Name:METHYL 1-(4-NITROPHENYL)-4-PIPERIDINECARBOXYLATE
- CAS Number:7595-60-0
- Molecular formula:C13H16N2O4
- Molecular Weight:264.28
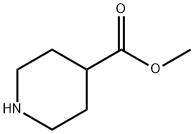
2971-79-1
283 suppliers
$10.00/10g
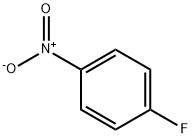
350-46-9
514 suppliers
$10.60/1gm:

7595-60-0
18 suppliers
inquiry
Yield:7595-60-0 85%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 80; for 10 h;
Steps:
1
To a stirred solution of 4-fluoro nitrobenzene (la, 3.1 g, 22 mmol) and methyl 4-piperidine carboxylate (2, 3.15 g, 22 mmol) in DMF solvent and K2CO3 (7.6 g, 55 mmol) as base and heated at 80 °C for 10h, after completion of the reaction, reaction is poured into ice water and extracted into ethyl acetate finally purification by column chromatography to afford pure compound methyl 1-(4-nitrophenyl)-4-piperidinecarboxylate (3a, 4.93 g, 85%). To a stirred solution of ester (3a, 4.75 g, 18 mmol) in ethanol, NH2NH2. H2O (2.25 g, 45 mmol) is added and refluxed for 24h. After completion of the reaction ethanol is evaporated under vaccum and water is added and extracted into ethyl acetate finally purification by column chromatography to afford pure compound 1-(4- nitrophenyl)-4-piperidinecarbohydrazide (4a, 3.99 g, 84%). Addition N,N-dimethyl carbamyl chloride (1.29 g, 12 mmol) to hydrazide (4a, 3.17 g, 12 mmol) in pyridine at room temperature (27 °C) and fallowed by reflux at temperature 85 °C for 2.5h. After completion of the reaction, the reaction mixture is cooled and filtered. The residue is recrystallized from water to get 5-[1 -(4-nitrophenyl)-4-piperidyl]-2,3-dihydro- 1,3,4- oxadiazol-2-one (Sa, 1.39 g, 40%). Nitro compound (5a, 1.16 g, 4 mmol) on reduction with SnCl2.2H2O (2.71 g, 12 mmol) in methanol and refluxed at 65 °C for 4h, after completion of reaction methanol is evaporated under vaccum and to this saturated sodium bicarbonate solution is added to quench the excess stannous chloride and filtered through celite bed and purified in silica column (60-120) to afforded pure compound 5-[1-(4- aminophenyl)-4-piperidyl]-2,3-dihydro-1,3,4-oxadiazol-2-one (7a, 884 mg, 85%). To a stirred solution of 5-nitro2-furanoic acid (0.16 g, 1 mmol) in DMF add HOBT (Hydroxybenzotriazole) (0.14 g, 1 mmol), EDCI (1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide)) (0.19 g, 1 mmol) and amine compound (7a, 0.26 g, 1 mmol) and stirred for 2h at room temperature (27 °C), after completion of the reaction, reaction mixture is poured into ice water and extracted into chloroform finally purification by column chromatography using ethyl acetate-hexane (7:3) as eluant to affordpure compound N2-4-[4-(5-oxo-4,5-dihydro-1,3,4-oxadiazol-2-yl)piperidino]phenyl-5-nitro-2- furamide (8a, 323 mg, 81%). 1H NMR (CDCl3, 300 MHz): δ 1.84-1.97 (m, 2H), 2.05-2.13 (m, 2H), 2.67-2.75 (m, 1H), 2.82-2.91 (m, 2H), 3.64-3.69 (m, 2H), 6.92 (d, 2H, J= 9.06 Hz), 7.34 (d, 1H, J=3.77 Hz), 7.38 (d, 1H, J= 3.77 Hz), 7.53 (d, 1H, J=9.06 Hz), 8.23 (bs, 1H); MS (ESI): m/z (400) (M+1)+.
References:
WO2013/93940,2013,A1 Location in patent:Page/Page column 15; 16