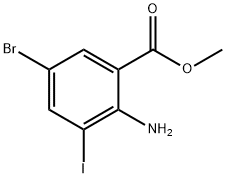
METHYL 2-AMINO-5-BROMO-3-IODOBENZOATE synthesis
- Product Name:METHYL 2-AMINO-5-BROMO-3-IODOBENZOATE
- CAS Number:289039-83-4
- Molecular formula:C8H7BrINO2
- Molecular Weight:355.96
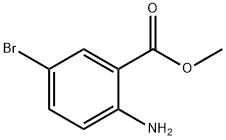
52727-57-8

289039-83-4
General procedure for the synthesis of methyl 2-amino-5-bromo-3-iodobenzoate from methyl 5-bromoaminobenzoate: 371 mg of methyl 2-amino-5-bromobenzoate (2 mmol) was dissolved in 2 mL of concentrated acetic acid, and 495 mg of N-iodosuccinimide (2.2 mmol) was added and the reaction was carried out for 17 hours at room temperature. After completion of the reaction, the mixture was poured into a mixture of 5 mL of saturated sodium bicarbonate solution and ice and extracted twice with ethyl acetate (EtOAc). The organic phases were combined, washed with saturated brine, dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure to give 585 mg of methyl 2-amino-5-bromo-3-iodobenzoate (82% yield) as a light brown solid. Mass spectrometry (EI) showed a molecular ion peak m/z 354.9 (M+).

52727-57-8
238 suppliers
$5.00/1g

289039-83-4
59 suppliers
$262.00/1g
Yield: 82%
Reaction Conditions:
with N-iodo-succinimide;acetic acid at 20; for 17 h;
Steps:
S9
Example S9; Preparation of 5-bromo-1H-indole-7-carboxylic acid; 371 mg of 2-amino-5-bromo-benzoic acid methyl ester (2 mmol) was dissolved in 2 ml conc acetic acid and treated with 495 mg of N-iodosuccinimide (2.2 mmol) at rt for 17 h. The reaction mixture was then poured on 5 ml saturated sodium bicarbonate solution and ice, extracted twice with EtOAc. The combined organic phases were then washed with brine, dried over magnesium sulfate, filtered and concentrated in vacuo, leading to 585 mg 2-amino-5-bromo-3-iodo-benzoic acid methyl ester (82%) as a light brown solid. MS (EI) 354.9 (M)+.
References:
Conte-Mayweg, Aurelia;Kuehne, Holger;Luebbers, Thomas;Maugeais, Cyrille;Mueller, Werner;Pflieger, Philippe US2006/30613, 2006, A1 Location in patent:Page/Page column 21-22