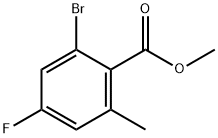
Methyl 2-broMo-4-fluoro-6-Methylbenzoate synthesis
- Product Name:Methyl 2-broMo-4-fluoro-6-Methylbenzoate
- CAS Number:1262396-04-2
- Molecular formula:C9H8BrFO2
- Molecular Weight:247.06

1003709-47-4

74-88-4

1262396-04-2
The general procedure for the synthesis of methyl 2-bromo-4-fluoro-6-methylbenzoate from 2-bromo-4-fluoro-6-methylbenzoic acid and iodomethane was as follows: 2-bromo-4-fluoro-6-methylbenzoic acid (1.94 g, 8.33 mmol), anhydrous potassium carbonate (1.72 g, 12.5 mmol), and iodomethane (2.36 g, 17 mmol) were mixed in N,N- dimethylformamide (15 mL) at 20°C with vigorous stirring for 23 hours. After completion of the reaction, the suspension was poured into 70 mL of water for liquid-liquid separation. The product was extracted with ethyl acetate (4 × 25 mL), and the combined organic phases were washed sequentially with water (5 × 20 mL) and brine (2 × 20 mL). The organic phase was dried over anhydrous sodium sulfate, filtered and concentrated to give 2.07 g (100% yield) of methyl 2-bromo-4-fluoro-6-methylbenzoate. The structure of the product was confirmed by 1H NMR (400 MHz, CDCl3): δ 7.18 (dd, 3JH-F = 8.1 Hz, 4JH-H = 2.4 Hz, 1H, Ar), 6.91 (dd, 3JH-F = 9.0 Hz, 4JH-H = 2.2 Hz, 1H, Ar), 3.96 (s, 3H, OCH3), 2.35 (s. 3H, CH3).

1003709-47-4
61 suppliers
$30.00/100mg

74-88-4
356 suppliers
$15.00/10g

1262396-04-2
42 suppliers
$34.00/250mg
Yield:1262396-04-2 100%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 20; for 23 h;
Steps:
1.e
Step eMethyl 2-bromo-4-fluoro-6-methylbenzoateA mixture of 2-bromo-4-fluoro-6-methylbenzoic acid (1.94 g, 8.33 mmol), anhydrous potassium carbonate (1.72 g,12.5 mmol), iodomethane (2.36 g, 17 mmol) in N,N-dimethylformamide (15 mL) was vigorously stirred for 23 hours at20 0C. The suspension was poured into 70 mL of water. A heavy liquid separated. The product was extracted with ethyl acetate (4 x 25 mL). The organic phase was washed with water (5 x 20 mL), brine (2 x 20 mL), dried with sodium sulfate, filtered and concentrated to give 2.07 g (100 %) of pure ester. 1H NMR (400 MHz, CDCI3) δ ppm 7.18(dd, 3JH-F = 8.1 Hz, 4JH-H = 2.4 Hz, 1H, Ar), 6.91 (dd, 3JH-F = 9.0 Hz, 4JH-H = 2.2 Hz, 1H, Ar), 3.96 (s, 3H, OCH3), 2.35(s, 3H, CH3).
References:
WO2011/6803,2011,A1 Location in patent:Page/Page column 35-36
452-71-1
274 suppliers
$12.00/25g

1262396-04-2
42 suppliers
$34.00/250mg
202865-77-8
128 suppliers
$9.00/1g

1262396-04-2
42 suppliers
$34.00/250mg
916792-09-1
73 suppliers
inquiry

1262396-04-2
42 suppliers
$34.00/250mg
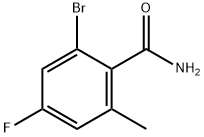
1262396-02-0
1 suppliers
inquiry

1262396-04-2
42 suppliers
$34.00/250mg