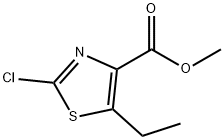
METHYL 2-CHLORO-5-ETHYLTHIAZOLE-4-CARBOXYLATE synthesis
- Product Name:METHYL 2-CHLORO-5-ETHYLTHIAZOLE-4-CARBOXYLATE
- CAS Number:1219632-45-7
- Molecular formula:C7H8ClNO2S
- Molecular Weight:205.66
28942-54-3
39 suppliers
$40.00/250mg

1219632-45-7
9 suppliers
inquiry
Yield:1219632-45-7 85%
Reaction Conditions:
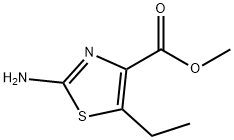
Stage #1: methyl 2-amino-5-ethyl-1,3-thiazole-4-carboxylatewith copper(ll) sulfate pentahydrate;sulfuric acid;sodium chloride;sodium nitrite in water at 0 - 20; for 1.75 h;
Stage #2: with copper(l) chloride in water at 20; for 16 h;
Steps:
172.a
The following diazotization step was adapted from Barton, A. et al, J. C. S. Perkin Trans I, 159-164 (1982): A solution of NaN02 (166 mg, 2.4 mmol) in water (0.6 mL) was added slowly to a stirred, cold (0 °C) solution of methyl 2-amino-5-ethyl-l,3- thiazole-4-carboxylate (186 mg, 1.0 mmol), CuS04*5H2O (330 mg, 1.32 mmol), NaCl (260 mg, 4.45 mmol) and H2S04 (5.5 mL) in water (7.5 mL). The mixture was stirred at 0 °C for 45 min and allowed to warm up to room temperature where it stirred further for 1 h before CuCl (1 18 mg) was added. This mixture was stirred further at room temperature for 16 h before it was diluted with brine and extracted with ether twice. The organic layers were combined, dried over MgS04 and concentrated to give methyl 2-chloro-5- ethylthiazole-4-carboxylate (i.e., Cap-172, step a) (175 mg, 85%) as an orange oil (80% pure) which was used directly in the next reaction. Rt = 1.99 min (Cond.-MDl); LC/MS: Anal. Calcd. for [M+H]+ C7H9C1N02S: 206.01 ; found: 206.05.
References:
WO2011/59850,2011,A1 Location in patent:Page/Page column 126-127