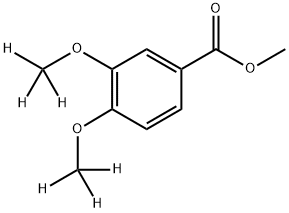
methyl 3,4-di[C2H3]methoxybenzoate synthesis
- Product Name:methyl 3,4-di[C2H3]methoxybenzoate
- CAS Number:1006717-12-9
- Molecular formula:C10H12O4
- Molecular Weight:196.2
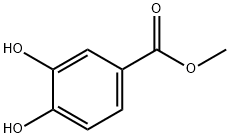
2150-43-8
290 suppliers
$13.43/1gm:
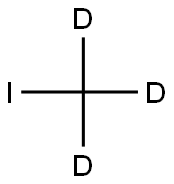
865-50-9
155 suppliers
$25.00/1g
![methyl 3,4-di[C2H3]methoxybenzoate](/CAS/20211123/GIF/1006717-12-9.gif)
1006717-12-9
3 suppliers
inquiry
Yield:1006717-12-9 97%
Reaction Conditions:
Stage #1: 3,4-dihydroxybenzoic acid methyl esterwith potassium carbonate in acetonitrile at 20; for 0.25 h;
Stage #2: iodomethane-d3 in acetonitrile at 45; for 15 h;
Steps:
3.1
Step 1. Methyl 3,4-bis(trideuteromethoxy)benzoate (15): To a suspension of commercially available methyl 3,4-dihydroxybenzoate (14, 2.0 g, 11.89 mmol) in acetonitrile (50 mL), was added potassium carbonate (4.9 g, 35.67 mmol). The mixture was stirred vigorously at ambient temperature for 15 minutes. To this reaction mixture was added iodomethane-d3 (2.4 mL, 38.06 mmol; Aldrich, 99.5 atom %D) and then the mixture was heated to 45 °C for a period of 15 hours. The mixture was then cooled to ambient temperature, filtered through Celite, and concentrated in vacuo. The crude residue was diluted with EtOAc (100 mL) and water (100 mL) and the resulting aqueous layer was further extracted with EtOAc (3 x 20 mL). The combined organic layers were washed with 10% aqueous Na2C03 (2 x 20 mL), dried (MgS04), filtered, andconcentrated in vacuo to afford ester 15 (2.3 g, 97%). MS (M+H): 203.2.
References:
WO2011/116066,2011,A1 Location in patent:Page/Page column 31-32

2150-43-8
290 suppliers
$13.43/1gm:
![methyl 3,4-di[C2H3]methoxybenzoate](/CAS/20211123/GIF/1006717-12-9.gif)
1006717-12-9
3 suppliers
inquiry