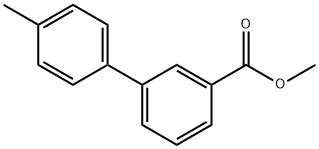
Methyl 3-(4-Methylphenyl)benzoate synthesis
- Product Name:Methyl 3-(4-Methylphenyl)benzoate
- CAS Number:114772-33-7
- Molecular formula:C15H14O2
- Molecular Weight:226.27
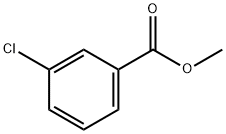
2905-65-9
150 suppliers
$14.00/5g
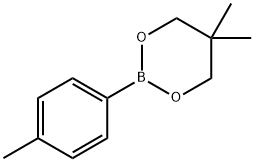
380481-66-3
77 suppliers
$25.00/1g

114772-33-7
22 suppliers
$55.00/1g
Yield:114772-33-7 97%
Reaction Conditions:
with potassium phosphate tribasic hydrate;NiIICl(1-naphthyl)(tricyclohexylphosphine)2;tricyclohexylphosphine in tetrahydrofuran at 23; for 4 h;Inert atmosphere;Glovebox;Sealed tube;Suzuki-Miyaura Coupling;
Steps:
Cross-Coupling of ortho-, meta-, and para-Substituted, Electron-Rich and Electron-Deficient Aryl Halides and Aryl Mesylates with Aryl Neopentylglycolboronates Catalyzed by NiIICl(1-naph-thyl)(PCy3)2/PCy3 in Anhydrous THF at 23 °C; General Procedure 2
General procedure: Cross-Coupling of ortho-, meta-, and para-Substituted, Electron-Rich and Electron-Deficient Aryl Halides and Aryl Mesylates with Aryl Neopentylglycolboronates Catalyzed by NiIICl(1-naph-thyl)(PCy3)2/PCy3 in Anhydrous THF at 23 °C; General Procedure 2In an oven-dried test tube charged with a Teflon coated stirring barwere added aryl halide or aryl mesylate (0.3 mmol), aryl neopentyl-glycolboronates (0.315 mmol), K3PO4(H2O)3.2 (191.00 ± 1.00 mg, 0.9mmol), and NiIICl(1-naphthyl)(PCy3)2 (11.73 ± 0.0510 mg, 0.015mmol, 5% catalyst loading). The test tube was brought into a N2 filledglove box (moisture level <2 ppm) through three degassing cycles andPCy3 (8.4 mg, 0.03 mmol, 10% loading) ligand was added. Distilled sol-vent (1 mL) was added inside the glove box and the test tube wassealed by a rubber septum and left stirring at 23 °C. A sample was tak-en by syringe and transferred outside the glove box. The sample wasdiluted by distilled THF (0.2 mL) and filtered through a short columnof Al2O3. The filtrate was concentrated and the GC analysis was car-ried out. The reaction mixture was diluted with CH2Cl2 (2 mL), filteredthrough a layer of Al2O3, and washed with CH2Cl2 (3 1 mL). The fil-trate was collected and concentrated under vacuum. The crude prod-uct was purified by column chromatography on silica gel with EtO-Ac/hexane mixture as eluent. The reductive elimination side-productwas also isolated and characterized.
References:
Malineni, Jagadeesh;Jezorek, Ryan L.;Zhang, Na;Percec, Virgil [Synthesis,2016,vol. 48,# 17,art. no. SS-2016-C0279-ST,p. 2795 - 2807] Location in patent:supporting information
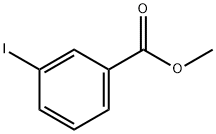
618-91-7
139 suppliers
$11.00/5g
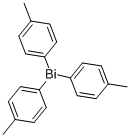
5142-75-6
32 suppliers
$191.00/1g

114772-33-7
22 suppliers
$55.00/1g

2905-65-9
150 suppliers
$14.00/5g
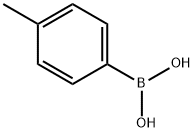
5720-05-8
449 suppliers
$5.00/1g

114772-33-7
22 suppliers
$55.00/1g
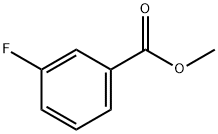
455-68-5
149 suppliers
$14.00/5g

380481-66-3
77 suppliers
$25.00/1g

114772-33-7
22 suppliers
$55.00/1g
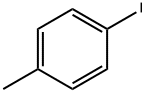
624-31-7
437 suppliers
$9.00/25g

618-91-7
139 suppliers
$11.00/5g

114772-33-7
22 suppliers
$55.00/1g