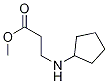
Methyl 3-(cyclopentylamino)propanoate synthesis
- Product Name:Methyl 3-(cyclopentylamino)propanoate
- CAS Number:754125-43-4
- Molecular formula:C9H17NO2
- Molecular Weight:171.2368
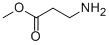
4138-35-6

120-92-3

754125-43-4
Step 2: Synthesis of methyl 3-(cyclopentylamino)propionate; Methyl 3-aminopropionate (9.37 g, 0.09 mol) was dissolved in dichloromethane (DCM, 200 mL), and cyclopentanone (6.43 mL, 0.07 mol), sodium acetate (5.96 g, 0.07 mol), and sodium triacetoxyborohydride (22 g, 0.10 mol) were added in turn . The reaction mixture was stirred at room temperature for 16 hours. After completion of the reaction, 20% sodium bicarbonate solution (100 mL) and 2M sodium hydroxide solution (50 mL) were added and separated by extraction with dichloromethane/water (DCM/H?O). The organic phases were combined, washed with saturated sodium chloride (NaCl) solution, dried with anhydrous magnesium sulfate (MgSO?), filtered, and the filtrate was concentrated under reduced pressure and then dried under vacuum to obtain the light yellow oily target product methyl 3-(cyclopentylamino)propionate (8.90 g, 55% yield); the molecular ion peak was detected in the positive mode of mass spectrometry (MS) m/z: 172.4.
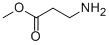
4138-35-6
47 suppliers
inquiry

120-92-3
427 suppliers
$11.00/25g

754125-43-4
40 suppliers
$37.00/250mg
Yield: 55%
Reaction Conditions:
Stage #1:methyl 3-aminopropanoate;cyclopentanone with sodium acetate;sodium tris(acetoxy)borohydride in dichloromethane at 20; for 16 h;
Stage #2: with sodium hydroxide;water;sodium hydrogencarbonate in dichloromethaneProduct distribution / selectivity;
Steps:
1.2; 2.1.2
Step 2: Methyl 3-(cyclopentylamino)propanoate; Methyl 3-aminopropanoate (9.37 g, 0.09 mol) was solubilised in DCM (200 ml) and cyclopentanone (6.43 ml, 0.07 mol), sodium acetate (5.96 g, 0.07 mol) and sodium triacetoxyborohydride (22 g, 0.10 mol) were added. The reaction was stirred at rt for 16h. 20% sodium bicarbonate (100 ml) and 2M sodium hydroxide (50 ml) were then added and the product extracted using DCM/η2O. The organic extracts were combined, washed with sat NaCl, dried using MgSO4, filtered and the filtrate evaporated under reduced pressure and further dried in vacuo to give the product as a pale yellow oil (8.90 g, 55%); MS+ve: 172.4.
References:
CYCLACEL LIMITED WO2009/40556, 2009, A1 Location in patent:Page/Page column 44; 75
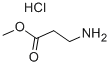
3196-73-4
343 suppliers
$6.00/5g

120-92-3
427 suppliers
$11.00/25g

754125-43-4
40 suppliers
$37.00/250mg
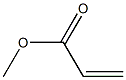
292638-85-8
5 suppliers
inquiry
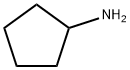
1003-03-8
236 suppliers
$10.00/1g

754125-43-4
40 suppliers
$37.00/250mg