METHYL-(3-HYDROXYPROPYL) SULFONE synthesis
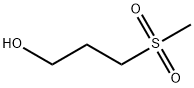
- Product Name:METHYL-(3-HYDROXYPROPYL) SULFONE
- CAS Number:2058-49-3
- Molecular formula:C4H10O3S
- Molecular Weight:138.19

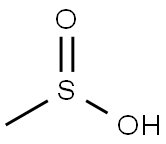
505-10-2

2058-49-3
General procedure for the synthesis of 3-methylsulfonyl-1-propanol from 3-methylthiopropanol: 3-methylthiopropanol (200 g, 1900 mmol) was dissolved in dichloromethane (2000 ml). The reaction mixture was cooled to 0°C. An 85% aqueous solution of m-chloroperoxybenzoic acid (970 g, 5700 mmol) was added in batches while maintaining the temperature between 0 and 5 °C. After the addition was completed, the reaction mixture was slowly warmed to 25 °C and the reaction was stirred at this temperature for 15 hours. After completion of the reaction, the mixture was filtered through a diatomaceous earth pad. The filtrate was purified by fast column chromatography (eluent: petroleum ether/ethyl acetate = 3:1, followed by ethyl acetate/methanol = 10:1) to afford the target product 3-methylsulfonyl-1-propanol (75 g, 29% yield).
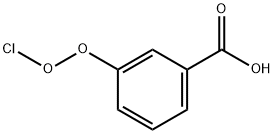
64741-01-1
0 suppliers
inquiry

505-10-2
350 suppliers
$5.00/10g
7757-83-7
772 suppliers
$13.50/250G

2058-49-3
69 suppliers
$15.00/1g
Yield: 66%
Reaction Conditions:
in dichloromethane;water
Steps:
11 EXAMPLE 11
3-Chloroperoxybenzoic acid (25 g, 97.2 mmol) was added in portions to a solution of 3-(methylsulphanyl)-1-propanol (5 ml, 48.6 mmol) in methylene chloride (100 ml) while maintaining the reaction temperature at 25° C. After stirring for 1 hour at ambient temperature, the solid was removed by filtration and the filtrate was diluted with an aqueous solution of sodium sulphite (6.5 g, 51.6 mmol) in water (200 ml). The organic layer was separated and the volatiles were removed by evaporation. The white residue was triturated with acetone and the volatiles were again removed by evaporation. The solid was then dissolved in methylene chloride and aluminum oxide (90 Å mesh) was added. After standing for 15 minutes, the solid was removed by filtration and the volatiles were removed by evaporation to give 3-(methylsulphonyl)-1-propanol as a colourless oil (4.46 g, 66%). 1H NMR Spectrum: (CDCl3) 1.9-2.1(br s, 1H); 2.15(m, 2H); 2.95(s, 3H); 3.2(t, 2H); 3.85(t, 2H) MS-EI: 139 [MH]+
References:
Zeneca Limited US6294532, 2001, B1

505-10-2
350 suppliers
$5.00/10g

2058-49-3
69 suppliers
$15.00/1g