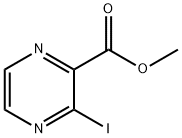
Methyl 3-iodopyrazine-2-carboxylate synthesis
- Product Name:Methyl 3-iodopyrazine-2-carboxylate
- CAS Number:173290-17-0
- Molecular formula:C6H5IN2O2
- Molecular Weight:264.02
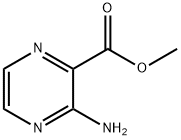
16298-03-6

173290-17-0
General procedure for the synthesis of pyrazine-2-iodo-3-carboxylic acid methyl ester from 3-aminopyrazine-2-carboxylic acid methyl ester: Referring to the method of Example 15, isopentyl nitrite (5.2 mL, 39 mmol) was slowly added to a suspension of 3-aminopyrazine-2-carboxylic acid methyl ester (1.9 g, 12 mmol) in diiodomethane (20 mL). Subsequently, the reaction mixture was heated to 85 °C and stirred continuously at 100 °C for 15 hours. After completion of the reaction, the mixture was cooled to room temperature and left to stand. The crude product was purified by silica gel column chromatography to afford 1.4 g of the target product 2-iodo-3-carboxylic acid methyl pyrazine as a light yellow solid in 44% yield. The product was characterized by 1H-NMR (500 MHz, CDCl3): δ 4.04 (s, 3H), 8.47 (d, J = 2.1 Hz, 1H), 8.56 (d, J = 2.1 Hz, 1H).

16298-03-6
335 suppliers
$7.00/1g

173290-17-0
87 suppliers
$12.00/100mg
Yield: 44%
Reaction Conditions:
with diiodomethane;isopentyl nitrite in diiodomethane at 85 - 100; for 15 h;
Steps:
15 3-Iodopyrazine-2-carboxylic acid methyl ester (Reference compound No.15-1)
Reference Example 15 3-Iodopyrazine-2-carboxylic acid methyl ester (Reference compound No.15-1) Isoamyl nitrite (5.2 mL, 39 mmol) was added to a suspension of 3-aminopyrazine-2-carboxylic acid methyl ester (1.9 g, 12 mmol) in diiodomethane (20 mL) at 85°C, then the mixture was stirred at 100°C for 15 hours. The reaction mixture was allowed to stand and purified by silica gel column chromatography to give 1.4 g of the title reference compound as a pale yellow solid. (Yield 44%) 1H-NMR(500MHz,CDCl3) δ 4.04(s,3H),8.47(d,J = 2.1 Hz,1H),8.56(d,J = 2.1 Hz,1H)
References:
SANTEN PHARMACEUTICAL CO., LTD. EP1602647, 2005, A1 Location in patent:Page/Page column 58