
METHYL 3'-NITRO[1,1'-BIPHENYL]-3-CARBOXYLATE synthesis
- Product Name:METHYL 3'-NITRO[1,1'-BIPHENYL]-3-CARBOXYLATE
- CAS Number:149506-24-1
- Molecular formula:C14H11NO4
- Molecular Weight:257.24
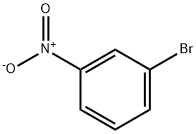
585-79-5
24 suppliers
$15.00/1g
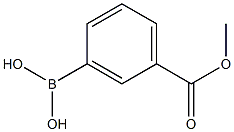
99769-19-4
265 suppliers
$10.00/1g
![METHYL 3'-NITRO[1,1'-BIPHENYL]-3-CARBOXYLATE](/StructureFile/ChemBookStructure8/GIF/CB6741903.gif)
149506-24-1
17 suppliers
$55.00/100mg
Yield:149506-24-1 65%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);sodium carbonate in 1,4-dioxane; for 16 h;Inert atmosphere;Reflux;Suzuki-Miyaura Coupling;
Steps:
4.3.1 1H NMR and 13C NMR spectra of Methyl 3′-nitro-[1,1′-biphenyl]-3-carboxylate (9)
1-Bromo-3-nitrobenzene 7 (5.0g, 24.7mmol), (3-(Methoxycarbonyl)phenyl)boronic acid 8 (5.34g, 29.7mmol), tetrakistriphenylphosphinepalladium(0) (1.40g, 1.23mmol), sodium carbonate (2M) (37mL, 74.1mmol), and 1,4-dioxane (120mL) were combined and the reaction mixture was heated at reflux under a nitrogen atmosphere for 16h. The reaction mixture was allowed to cool at room temperature and partitioned between ethyl acetate and water. The organic phase was separated, dried over magnesium sulfate, filtered, and the filtrate was concentrated to give a liquid. The crude product was purified by flash chromatography over silica with a hexanes:ethyl acetate gradient (100:0 to 90:10) to give (4.12g, 65%) the title compound 9 as a yellow solid; m.p.: 96.5-96.6°C; 1H NMR (400MHz, DMSO-d6): δ 8.45 (t, J=4.00Hz, 1H), 8.27-8.24 (m, 2H), 8.19 (dd, J=4.00, 8.00Hz, 1H), 8.08-8.01 (m, 2H), 7.79 (t, J=8.00Hz, 1H), 7.68 (t, J=8.00Hz, 1H), 3.90 (s, 3H),; 13C NMR (100MHz, DMSO-d6): δ 166.4, 148.9, 141.0, 138.8, 133.9, 132.3, 131.1, 131.0, 130.2, 129.6, 127.9, 123.1, 121.8, 52.8; IR (ATR): νmax 3312, 2953, 2342, 1722, 1520, 1429, 1348, 1301, 1236, 1191, 1104, 963, 883, 839, 801, 690; HRMS (ESI): m/z calcd for C14H11NO4 Na [M+ Na]+: 280.0586; found: 280.0579.
References:
Kuppusamy, Rajesh;Yasir, Muhammad;Berry, Thomas;Cranfield, Charles G.;Nizalapur, Shashidhar;Yee, Eugene;Kimyon, Onder;Taunk, Aditi;Ho, Kitty K.K.;Cornell, Bruce;Manefield, Mike;Willcox, Mark;Black, David StC;Kumar, Naresh [European Journal of Medicinal Chemistry,2018,vol. 143,p. 1702 - 1722]
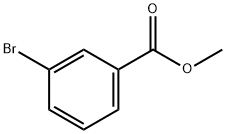
618-89-3
271 suppliers
$29.00/5g
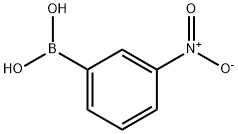
13331-27-6
353 suppliers
$5.00/1g
![METHYL 3'-NITRO[1,1'-BIPHENYL]-3-CARBOXYLATE](/StructureFile/ChemBookStructure8/GIF/CB6741903.gif)
149506-24-1
17 suppliers
$55.00/100mg