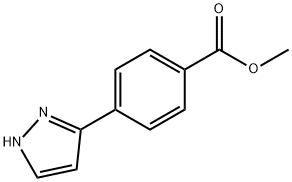
METHYL 4-(1H-PYRAZOL-3-YL) BENZOATE synthesis
- Product Name:METHYL 4-(1H-PYRAZOL-3-YL) BENZOATE
- CAS Number:179057-10-4
- Molecular formula:C11H10N2O2
- Molecular Weight:202.21
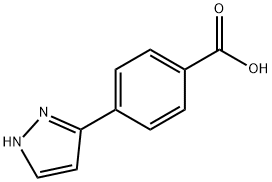
208511-67-5

74-88-4

179057-10-4
4-(1H-pyrazol-3-yl)benzoic acid (0.30 g, 1.6 mmol) and iodomethane (0.34 g, 2.4 mmol) were used as raw materials, and the reaction was stirred for 1 h at room temperature with the addition of K2CO3 (0.66 g, 7.78 mmol) in DMF (8 mL) (the progress of the reaction was monitored by TLC, and the unfolding agent was 10% MeOH/CHCl3). . After completion of the reaction, the mixture was diluted with water (30 mL) and extracted with EtOAc (2 x 30 mL). The organic layers were combined, washed sequentially with water and brine, dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure to afford methyl 4-(1H-pyrazol-3-yl)benzoate (0.31 g, 96% yield). The product was characterized by 1H NMR (DMSO-d6): δ 13.10 (br s, 1H), 7.98 (m, 4H), 7.80 (br s, 1H), 6.83 (d, J = 2.2 Hz, 1H), 3.86 (s, 3H).
![4-[(2E)-3-(Dimethylamino)-1-oxo-2-propen-1-yl]benzoic acid methyl ester](/CAS2/GIF/114431-72-0.gif)
114431-72-0
30 suppliers
$130.00/1g

179057-10-4
46 suppliers
$27.00/100mg
Yield:179057-10-4 8.15 g
Reaction Conditions:
with hydrazine hydrate in ethanol at 50 - 60; for 54 h;
Steps:
43 Example 43. Preparation of methy -(lH-pyrazol-3-yl)benzoate (B41)
Example 43. Preparation of methy -(lH-pyrazol-3-yl)benzoate (B41) To a solution of crude (i^-methyl 4-(3-(dimethylamino)acryloyl)benzoate (28.1 mmol) in EtOH (100 mL) was added hydrazine monohydrate (1.50 mL, 30.9 mmol) and the reaction was heated at 50 °C for 24 hours. The reaction temperature was then increased to 60 °C for 24 hours. Additional hydrazine monohydrate (1.5 mL) was added, and the reaction was heated at 60 °C for an additional 6 hours. The reaction was cooled, concentrated, and dried in a vacuum oven at 45 °C overnight to yield methyl 4-(lH-pyrazol-3-yl)benzoate as an orange solid (8.15 g, quantitative): mp 106 °C (dec); ]H NMR (400 MHz, CDC13) δ 8.15 - 8.05 (m, 2H), 7.91 - 7.83 (m, 2H), 7.65 (d, J = 2.4 Hz, 1H), 6.71 (d, J = 2.3 Hz, 1H), 3.94 (s, 3H); 1JC NMR (101 MHz, CDC13) δ 166.91 , 136.89, 131.83, 130.13, 129.37, 125.50, 103.35, 52.14, 22.46; EIMS m/z 202.
References:
WO2014/11429,2014,A1 Location in patent:Page/Page column 51
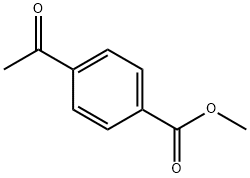
3609-53-8
208 suppliers
$5.00/1g

179057-10-4
46 suppliers
$27.00/100mg