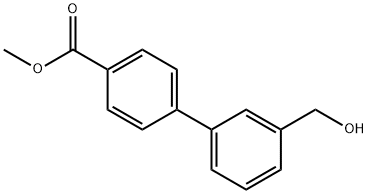
Methyl 4-(3-hydroxymethylphenyl)benzoate synthesis
- Product Name:Methyl 4-(3-hydroxymethylphenyl)benzoate
- CAS Number:223126-96-3
- Molecular formula:C15H14O3
- Molecular Weight:242.27
221021-36-9
42 suppliers
$29.00/250mg

223126-96-3
14 suppliers
$424.00/1g
Yield:-
Reaction Conditions:
with sodium borohydrid;acetic acid in ethanol;
Steps:
R.15.a (a)
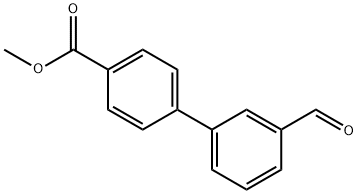
(a) Methyl 3'-hydroxymethylbiphenyl-4-carboxylate Sodium borohydride (132 mg) was added to a solution of methyl 3'-formylbiphenyl-4-carboxylate (720 mg), which is the product of Reference example 14(a), in ethanol (50 ml) at ambient temperature. The mixture was stirred under an atmosphere of nitrogen for 50 minutes. The reaction mixture was quenched with 50% acetic acid and concentrated under reduced pressure. The residue was partitioned between ethyl acetate and water and the layers were separated. The ethyl acetate layer was washed with saturated aqueous sodium chloride solution and dried over anhydrous magnesium sulfate and concentrated under reduced pressure. The residue was crystallized from diisopropyl ether to afford the desired compound (522 mg) as colorless crystals. mp 88-89° C. 1H-NMR (270 MHz, CDCl3): δ ppm 3.95 (3H, s), 4.79 (2H, s), 7.40 (1H, d, J=7.5 Hz), 7.47 (1H, t, J=7.5 Hz), 7.56 (1H, d, J=7.5 Hz), 7.64 (1H,s), 7.67 (2H, d, J=8.5 Hz), 8.11 (2H, d, J=8.5 Hz).
References:
US6528525,2003,B1