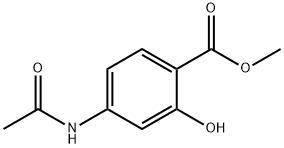
METHYL 4-ACETAMIDO-2-HYDROXYBENZOATE synthesis
- Product Name:METHYL 4-ACETAMIDO-2-HYDROXYBENZOATE
- CAS Number:4093-28-1
- Molecular formula:C10H11NO4
- Molecular Weight:209.2

75-36-5
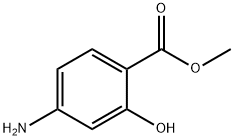
4136-97-4

4093-28-1
Methyl 4-amino-2-hydroxybenzoate (50.7 g, 303.6 mmol) was dissolved in ethyl acetate (750 mL) with stirred water (250 mL) and sodium bicarbonate solution (34.9 g). The mixture was cooled to 0°C over 15 minutes, followed by slow dropwise addition of acetyl chloride (29.7 mL, 415.5 mmol). The reaction mixture was gradually warmed to room temperature and stirred continuously for 2 hours. After completion of the reaction, the organic and aqueous layers were separated, the organic layer was washed with brine and dried over anhydrous sodium sulfate. The solvent was removed by concentration under reduced pressure to give the white solid product methyl 4-acetamido-2-hydroxybenzoate (63.5 g, 99% yield). The product was confirmed by NMR hydrogen spectrum (CDCl3): δ10.86 (broad single peak, 1H), 7.78 (double peak, J=8.6Hz, 1H), 7.23 (single peak, 1H), 7.16 (broad single peak, 1H), 7.10 (double peak, J=8.6Hz, 1H), 6.13 (broad single peak, 1H), 3.92 (single peak, 3H), 2.19 ( single peak, 3H); mass spectrum (m/z): 208 (MH)+.
Yield: 99%
Reaction Conditions:
with sodium hydrogencarbonate in water;ethyl acetate at 0 - 20; for 2.25 h;
Steps:
1.ii Step (ii)
Preparation of Methyl 4-acetylamino-2-hydroxy benzoate
A solution of Methyl 4-amino-2-hydroxy benzoate (50.7 grams, 303.6 mmol, obtained in above step) in ethyl acetate (750 mL) was added to a stirred solution of water (250 mL) and sodium bicarbonate (34.9 grams, 415.5 mmol) cooled at 0 °C followed by acetyl chloride (29.7 mL, 415.5 mmol) over a period of 15 minutes. The reaction mixture was gradually warmed to room temperature and stirred for 2 hours. The two layers were separated and the organic layer was washed with brine, dried over anhydrous sodium sulphate and the solvent was removed under reduced pressure to obtain methyl 4-acetylamino-2-hydroxy benzoate (63.5 grams).Yield: 99 %.Ή - NMR (CDC13): δ 10.86 (bs, 1H), 7.78 (d, J = 8.6 Hz, 1H), 7.23 (s, 1H), 7.16 (bs, 1H), 7.10 (d, J = 8.6 Hz, 1H), 6.13 (bs, 1H), 3.92 (s, 3H), 2.19 (s, 3H);Mass (m/z): 208 (M-H)+.
References:
SUVEN LIFE SCIENCES LIMITED;NIROGI, Ramakrishna;MOHAMMED, Abdul Rasheed;YARLGADDA, Suresh;RAVELLA, Srinivasa Rao;SHINDE, Anil Karbhari;KAMBHAMPATI, Ramasastri;ROAYALLEY, Praveen Kumar;JAYARAJAN, Pradeep;BHYRAPUNENI, Gopinadh;PATNALA, Sriramachandra Murthy;RAVULA, Jyothsna;JASTI, Venkateswarlu WO2013/42135, 2013, A1 Location in patent:Page/Page column 11
127-09-3
1177 suppliers
$8.00/1 ea

4136-97-4
186 suppliers
$6.00/5g

4093-28-1
148 suppliers
$6.00/5g

4136-97-4
186 suppliers
$6.00/5g

4093-28-1
148 suppliers
$6.00/5g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

4093-28-1
148 suppliers
$6.00/5g
