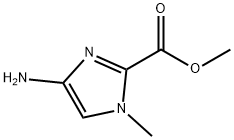
METHYL 4-AMINO-1-METHYL-1H-IMIDAZOLE-2-CARBOXYLATE synthesis
- Product Name:METHYL 4-AMINO-1-METHYL-1H-IMIDAZOLE-2-CARBOXYLATE
- CAS Number:162085-97-4
- Molecular formula:C6H9N3O2
- Molecular Weight:155.15
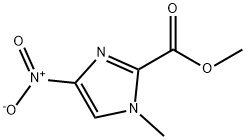
169770-25-6

162085-97-4
1. Methyl 1-methyl-4-nitro-1H-imidazole-2-carboxylate (3.3 g, 17.8 mmol) was dissolved in a 1:1 solvent mixture of methanol/ethyl acetate (100 mL) and Pd/C catalyst (400 mg) was added. The reaction was shaken at 70 psi hydrogen pressure for 14 hours. 2. Upon completion of the reaction, the reaction mixture was filtered through a bed of diatomite and the diatomite was washed with methanol. The filtrate was concentrated under reduced pressure to give the crude product methyl 4-amino-1-methyl-1H-imidazole-2-carboxylate as a black oil. 3. The crude product was dissolved in benzene and concentrated again under reduced pressure to obtain a black solid. It was filtered and washed with ether to give purified amine (2.5 g, 88%). 4. The purified amine (14.7 mmol) was dissolved in acetonitrile (50 mL) and N,N-diisopropylethylamine (DIEA, 2.3 mL, 18 mmol) was added. An acetonitrile solution (50 mL) of 1-methyl-2-trichloroacetylimidazole (3.6 g, 14.7 mmol) was added slowly at 0 °C. The reaction mixture was allowed to stand for 5 minutes at room temperature. 5. The reaction mixture was stirred at room temperature for 10 hours. After completion of the reaction, the solvent was removed under reduced pressure and the residue was dissolved in methanol and the product was precipitated by the addition of ether to afford the target compound, methyl 4-amino-1-methyl-1H-imidazole-2-carboxylate (2.13 g, 68%). 6. The structure of the product was confirmed by the presence of 1H-imidazole (3.6 g, 14.7 mmol). 6. The structure of the product was confirmed by 1H-NMR, 13C-NMR and EI-HRMS.
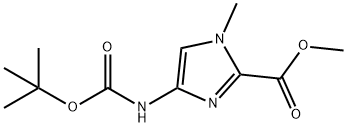
500701-36-0
15 suppliers
inquiry

162085-97-4
39 suppliers
inquiry
Yield:162085-97-4 160 mg
Reaction Conditions:
with trifluoroacetic acid in dichloromethane at 0;Inert atmosphere;
Steps:
17 Preparation of Compound 49
Compound 48 (300 mg, 1.17 mmol) was dissolved in dichloromethane (1 mL), then trifluoroacetic acid (1 mL)was added at 0°C under a nitrogen atmosphere, and the mixture was stirred for 2 hours. The reaction mixture wasconcentrated under reduced pressure, then diluted with dichloromethane (30 mL), and washed with saturated aqueoussodium hydrogen carbonate solution (30 mL). The aqueous layer was extracted with dichloromethane (30 mL), and thecollected organic layers were dried over anhydrous sodium sulfate. Filtration and concentration were performed, therebyobtaining Compound 49 (160 mg). 1H-NMR (400 MHz, CDCl3) δ 6.37 (s, 1H), 3.92 (s, 3H). 3.90 (s, 3H), 3.56 (brs, 2H)EI-MS m/z : [M+H]+156.17.
References:
EP4086268,2022,A1 Location in patent:Paragraph 0195; 0199

169770-25-6
68 suppliers
inquiry

162085-97-4
39 suppliers
inquiry
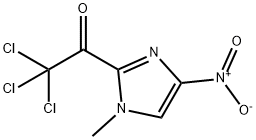
120095-64-9
36 suppliers
$95.00/250mg

162085-97-4
39 suppliers
inquiry
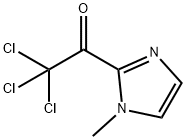
30148-23-3
55 suppliers
inquiry

162085-97-4
39 suppliers
inquiry