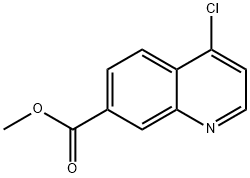
methyl 4-chloroquinoline-7-carboxylate synthesis
- Product Name:methyl 4-chloroquinoline-7-carboxylate
- CAS Number:178984-69-5
- Molecular formula:C11H8ClNO2
- Molecular Weight:221.64
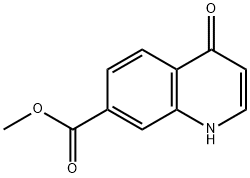
863785-96-0

178984-69-5
General procedure for the synthesis of methyl 4-chloroquinoline-7-carboxylate from methyl 4-oxo-1,4-dihydroquinoline-7-carboxylate: the residue (0.59 g) was dissolved in N,N-dimethylformamide (20 ml), triethylamine (4 ml) and 10% palladium/carbon (0.59 g) were added. The reaction mixture was stirred overnight under hydrogen atmosphere and at room temperature. After completion of the reaction, the mixture was filtered and the filter cake was washed with chloroform. The solvent was removed by distillation under reduced pressure and the residue was washed with methanol to afford methyl 4-oxo-1,4-dihydroquinoline-7-carboxylate (187 mg, 28% yield). The resulting methyl 4-oxo-1,4-dihydroquinoline-7-carboxylate (650 mg) was suspended in diisopropylethylamine (7 ml), phosphorus trichloride (1.5 ml) was added, and the reaction was stirred at 120 °C for 30 min. After completion of the reaction, water was added to the reaction mixture under ice bath cooling. The aqueous layer was neutralized with aqueous sodium bicarbonate and the organic layer was extracted with ethyl acetate. The ethyl acetate layer was washed with water and dried with anhydrous sodium sulfate. The solvent was removed by distillation under reduced pressure and the residue was purified by column chromatography in acetone-chloroform system to afford methyl 4-chloroquinoline-7-carboxylate (609 mg, 86% yield).

863785-96-0
42 suppliers
$155.00/100mg

178984-69-5
45 suppliers
$65.00/100mg
Yield: 86%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine;trichlorophosphate at 120; for 0.5 h;
Steps:
113
A part of the residue (0.59 g) was dissolved in N,N-dimethylformamide (20 ml). Triethylamine (4 ml) and 10% palladium/carbon (0.59 g) were added to the solution, and the mixture was stirred under a hydrogen gas atmosphere at room temperature overnight. The reaction mixture was filtered and was then washed with chloroform. The solvent was removed by distillation under the reduced pressure, and the residue was washed with methanol to give methyl 4-oxo-1,4-dihydro-quinoline-7-carboxylate (187 mg, yield 28%) (3 steps). Methyl 4-oxo-1,4-dihydro-quinoline-7-carboxylate (650 mg) was suspended in diisopropylethylamine (7 ml), phosphorus oxychloride (1.5 ml) was added to the suspension, and the mixture was stirred at 120°C for 30 min. Water was added to the reaction mixture under ice cooling. The aqueous layer was neutralized with an aqueous sodium hydrogencarbonate solution, and the organic layer was extracted with ethyl acetate. The ethyl acetate layer was then washed with water and was dried over anhydrous sodium sulfate. The solvent was removed by distillation under the reduced pressure, and the residue was purified by column chromatography with an acetone-chloroform system to give methyl 4-chloro-quinoline-7-carboxylate (609 mg, yield 86%).
References:
KIRIN BEER KABUSHIKI KAISHA EP1724268, 2006, A1 Location in patent:Page/Page column 76
49713-58-8
62 suppliers
$35.00/100mg

74-88-4
357 suppliers
$15.00/10g

178984-69-5
45 suppliers
$65.00/100mg
1374258-80-6
0 suppliers
inquiry

178984-69-5
45 suppliers
$65.00/100mg
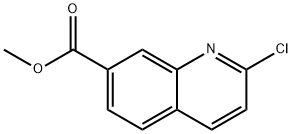
1416801-65-4
18 suppliers
inquiry
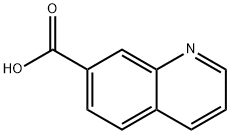
1078-30-4
134 suppliers
$20.00/100mg

178984-69-5
45 suppliers
$65.00/100mg
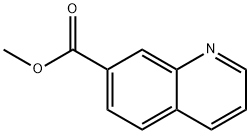
51552-68-2
48 suppliers
$41.00/100mg

178984-69-5
45 suppliers
$65.00/100mg