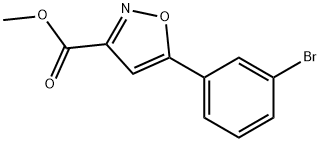
Methyl 5-(3-Bromophenyl)isoxazole-3-carboxylate synthesis
- Product Name:Methyl 5-(3-Bromophenyl)isoxazole-3-carboxylate
- CAS Number:745078-74-4
- Molecular formula:C11H8BrNO3
- Molecular Weight:282.09
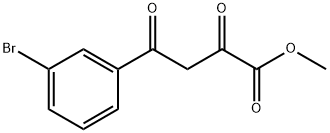
93618-22-5

745078-74-4
Methyl 2,4-dioxo-4-(3-bromophenyl)butyrate (10.3 g, 36.1 mmol) was used as a raw material and suspended in anhydrous methanol (100 mL). To this suspension was added hydroxylamine hydrochloride (3.8 g, 54.2 mmol). The reaction mixture was heated to reflux for 90 minutes. Upon completion of the reaction, the mixture was cooled to room temperature and an ice/water mixture (200 mL) was added. After continued stirring for 20 minutes, the mixture was filtered through a Brinell funnel and washed with cold water. The resulting light yellow solid was dried in a vacuum oven to give methyl 5-(3-bromophenyl)isoxazole-3-carboxylate (8.9 g, 87% yield).

93618-22-5
21 suppliers
$336.00/1g

745078-74-4
38 suppliers
$47.00/250mg
Yield:745078-74-4 87%
Reaction Conditions:
with hydroxylamine hydrochloride in methanol;
Steps:
2.B 5-(3-Bromo-phenyl)-isoxazole-3-carboxylic Acid Methyl Ester
Example 2B 5-(3-Bromo-phenyl)-isoxazole-3-carboxylic Acid Methyl Ester To a suspension of 2,4-dioxo-4-(3-bromophenyl)-butyric acid methyl ester (10.3 g, 36.1 mmol) in anhydrous MeOH (100 mL) was added hydroxylamine hydrochloride (3.8 g, 54.2 mmol). The mixture was then refluxed for 90 min. The reaction mixture was then cooled to room temperature, and ice/water mixture (200 mL) was added. The mixture was stirred for 20 minutes, filtered through a Buchner funnel and washed with cold water. The light yellow solid was dried in vacuum oven to provide the title compound (8.9 g, 87% yield).
References:
US2004/167188,2004,A1