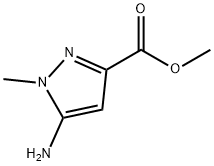
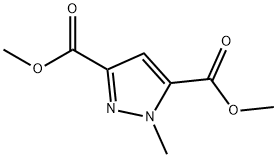
methyl 5-amino-1-methyl-1H-pyrazole-3-carboxylate synthesis
- Product Name:methyl 5-amino-1-methyl-1H-pyrazole-3-carboxylate
- CAS Number:92406-53-6
- Molecular formula:C6H9N3O2
- Molecular Weight:155.15
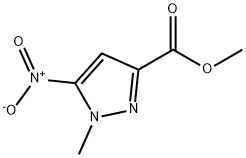
796038-07-8

92406-53-6
General procedure for the synthesis of methyl 5-amino-1-methylpyrazole-3-carboxylate from methyl 1-methyl-5-nitro-1H-pyrazole-3-carboxylate: Methyl 1-methyl-5-nitro-1H-pyrazole-3-carboxylate (136 mg, 0.74 mmol) obtained in Preparation Example 21 was dissolved in 2 mL of methanol, and 14 mg of 10% palladium/charcoal catalyst was slowly added dropwise. The reaction was stirred at room temperature for 1 hour under hydrogen pressure of 50 atm. Upon completion of the reaction, the reaction mixture was filtered through a pad of diatomaceous earth to remove the catalyst and the filtrate was concentrated under reduced pressure to afford 94 mg (82% yield) of the target compound, methyl 5-amino-1-methylpyrazole-3-carboxylate. The product was characterized by 1H NMR (300 MHz, CDCl3): δ 6.12 (s, 1H, pyrazole ring H), 3.99 (s, 3H, OCH3), 3.84 (s, 3H, N-CH3), 3.72 (brs, 2H, NH2).

796038-07-8
19 suppliers
inquiry

92406-53-6
64 suppliers
$85.00/100mg
Yield: 87%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in methanol at 20; under 760.051 Torr; for 4 h;
Steps:
P35.2 Step 2. Synthesis of methyl 5-amino-1-methyl-1H-pyrazol-3-carboxylate (60)
At room temperature, Compound 59 (200 mg, 1.081 mmol) was dissolved in methanol (10 mL) and added with Pd/C (10%, 90 mg). The reaction was stirred for 4 h under 1 atm of hydrogen gas. After the reaction was completed as monitored by LCMS, the reaction was filtered with suction. The filtrate was concentrated under reduced pressure to give methyl 5-amino-1-methyl-1H-pyrazol-3-carboxylate 60 (145 mg, light yellow oil). Yield: 87.0%. LCMS: m/z 156 (M+H).
References:
SHANGHAI HAIHE PHARMACEUTICAL CO., LTD.;SHANGHAI INSTITUTE OF MATERIA MEDICA, CHINESE ACADEMY OF SCIENCES;LI, Lei;GENG, Meiyu;HUANG, Ying;DING, Jian;ZHANG, Qiong;HUANG, Min;TANG, Shuai;SHEN, Ning;CHEN, Yi US2020/247781, 2020, A1 Location in patent:Paragraph 0426-0427

796038-07-8
19 suppliers
inquiry
177409-38-0
30 suppliers
inquiry
89088-56-2
40 suppliers
$56.00/100mg

92406-53-6
64 suppliers
$85.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
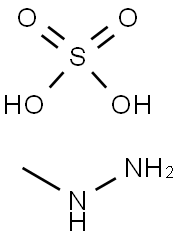
302-15-8
198 suppliers
$21.00/1g

92406-53-6
64 suppliers
$85.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

92406-53-6
64 suppliers
$85.00/100mg
33146-99-5
74 suppliers
$29.00/250mg

92406-53-6
64 suppliers
$85.00/100mg