
METHYL 5-CYANO-2-HYDROXY-3-IODOBENZOATE synthesis
- Product Name:METHYL 5-CYANO-2-HYDROXY-3-IODOBENZOATE
- CAS Number:952302-09-9
- Molecular formula:C9H6INO3
- Molecular Weight:303.05
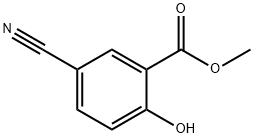
84437-12-7
73 suppliers
inquiry

952302-09-9
10 suppliers
inquiry
Yield:952302-09-9 41%
Reaction Conditions:
with chloramine-T;sodium iodide in N,N-dimethyl-formamide at 20 - 95;
Steps:
105.A A) Methyl 5-cyano-2-hydroxy-3-iodobenzoate
To a solution of methyl 5-cyano-2-hydroxybenzoate (3.0 g, 16.9 mmol) in N,N- dimethylformamide (30 mL) was added sodium iodide (3.05 g, 20.3 mmol), followed by chloramine T trihydrate (5.25 g, 18.6 mmol) slowly. The resulting mixture was stirred at room temperature for 2 hours, then heated at 95 °C overnight. The reaction mixture was diluted with ethyl acetate and 1 N aqueous HCl solution. The organic layer was separated, washed with brine followed by Na2S2O3solution, dried over MgSO4, and filtered. The filtrate was concentrated in vacuo to give a light yellow oil. To the residue was added MeOH and the solid was filtered to give the title compound as a white solid (2.1 g, 41%).1H NMR (400 MHz, CHLOROFORM-d) δ 12.19 (s, 1H), 8.22 - 8.14 (m, 2H), 4.05 (s, 3H).
References:
WO2022/169921,2022,A1 Location in patent:Page/Page column 180-181