
methyl 5-hydroxy-2-methyl-3-nitrobenzoate synthesis
- Product Name:methyl 5-hydroxy-2-methyl-3-nitrobenzoate
- CAS Number:88132-51-8
- Molecular formula:C9H9NO5
- Molecular Weight:211.17
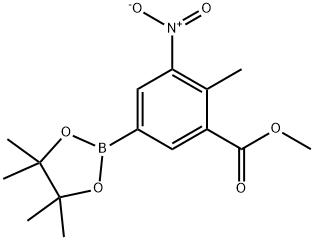
1547162-72-0
2 suppliers
inquiry

88132-51-8
23 suppliers
inquiry
Yield:88132-51-8 78%
Reaction Conditions:
with potassium peroxymonosulfate in water;acetone at 20; for 1 h;Cooling with ice;
Steps:
2 [001851 Preparation of methyl 5-hydroxy-2-methyl-3-nitrobenzoate C2-4.
[001851 Preparation of methyl 5-hydroxy-2-methyl-3-nitrobenzoate C2-4. To a solution of compound C2-3 (24.2 g, 68.5 mmol) in acetone (250 mL) in an ice-water bath was added sat. aq. OXONE (100 mL) with vigorous stirring. The mixture was stirred for another 1 hr at room temperature. The reaction was then quenched with sat. aq. NaHSO3. The mixture was concentrated in vacuo and extracted with EA. The combined organic layers were washed with brine, dried over MgSO4, concentrated, and purified by reversed phase chromatography to yield compound C2-4 as a grey-white solid (11.2 g, yield: 78%). ‘H NMR (CDC13, 400 MHz) : 7.42 (d, J 2.4 Hz, 1H), 7.37 (d, J= 2.4 Hz, 1H), 3.94 (s, 3H), 2.50 (s, 3H); MS (ESI): m/z 212 (M+1).
References:
WO2014/18866,2014,A1 Location in patent:Paragraph 00181; 00185
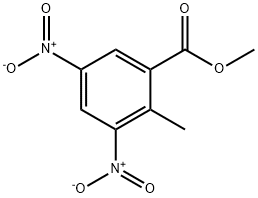
52090-24-1
64 suppliers
$28.60/10MG

88132-51-8
23 suppliers
inquiry
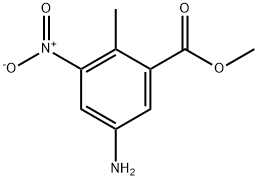
88132-48-3
14 suppliers
inquiry

88132-51-8
23 suppliers
inquiry
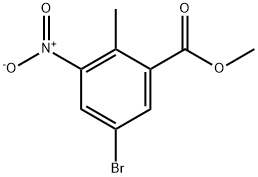
220514-28-3
151 suppliers
$10.00/1g

88132-51-8
23 suppliers
inquiry

88132-50-7
0 suppliers
inquiry

88132-51-8
23 suppliers
inquiry