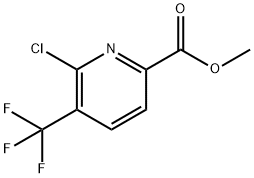
Methyl 6-chloro-5-(trifluoroMethyl)picolinate synthesis
- Product Name:Methyl 6-chloro-5-(trifluoroMethyl)picolinate
- CAS Number:1211518-35-2
- Molecular formula:C8H5ClF3NO2
- Molecular Weight:239.58
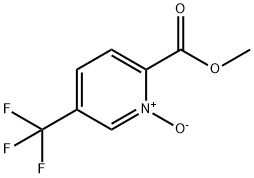
1415899-18-1

1211518-35-2
Methyl oxo-5-trifluoromethylpyridine-2-carboxylate (2.2 g, 10 mmol) was added batchwise to phosphoric acid trichloride (CAS 10025-87-3, 10 mL) at 0 °C, the reaction mixture was stirred and reacted at 50 °C overnight. Upon completion of the reaction, the solvent was removed by vacuum distillation to give a brown oil. The oily substance was dissolved in ethyl acetate (30 mL) and minimally neutralized with aqueous sodium carbonate. The neutralized mixture was extracted with ethyl acetate (2 x 30 mL), the organic phases were combined and washed with brine (30 mL), dried over anhydrous sodium sulfate, filtered and concentrated to give a light brown solid. Purification of the above solid by column chromatography (silica gel, 15 g, 3% petroleum ether solution of ethyl acetate) resulted in methyl 6-chloro-5-(trifluoromethyl)pyridinecarboxylate (1.5 g, 63% yield) as a white solid; mass spectrum (EI): m/e = 240.0 [M + H]+.

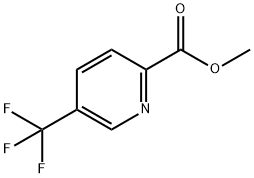
1415899-18-1
1 suppliers
inquiry

1211518-35-2
63 suppliers
$99.00/100mg
Yield:1211518-35-2 63%
Reaction Conditions:
with trichlorophosphate at 0 - 50;
Steps:
113.c
Oxy-5-trifluoromethyl-pyridine-2-carboxylic acid methyl ester (2.2 g, 10 mmol) was added in portions to phosphoryl trichloride (CAN 10025-87-3, 10 mL) at 0° C. and the resulting mixture was stirred at 50° C. overnight. Removal of the solvent in vacuo gave a brown oil which was dissolved in ethyl acetate (30 mL) and carefully neutralized with a aqueous solution of sodium carbonate. The mixture was extracted with ethyl acetate (2×30 mL) and the combined organic phase was washed with brine (30 mL), dried over anhydrous sodium sulfate, filtered and concentrated to give a light-brown solid. The solid was purified by column chromatography (silica gel, 15 g, 3% ethyl acetate in petroleum ether) to give the target compound (1.5 g, 63%) as white solid; MS (EI): m/e=240.0 [M+H]+.
References:
US2012/316147,2012,A1 Location in patent:Page/Page column 77
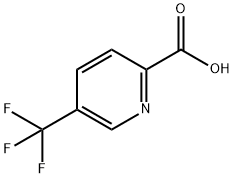
124236-37-9
127 suppliers
$13.00/250mg

1211518-35-2
63 suppliers
$99.00/100mg
80194-69-0
196 suppliers
$10.00/1g

1211518-35-2
63 suppliers
$99.00/100mg