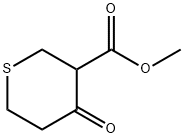
METHYL TETRAHYDRO-4-OXO-2H-THIOPYRAN-3-CARBOXYLATE synthesis
- Product Name:METHYL TETRAHYDRO-4-OXO-2H-THIOPYRAN-3-CARBOXYLATE
- CAS Number:4160-61-6
- Molecular formula:C7H10O3S
- Molecular Weight:174.22
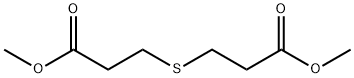
4131-74-2

4160-61-6
General procedure for the synthesis of methyl tetrahydro-4-oxo-2H-thiopyran-3-carboxylate from dimethyl 3,3'-thiodipropionate: 2.7098 g of NaH (60%) was added to a dry 250 ml three-necked flask, followed by the addition of 40 ml of anhydrous tetrahydrofuran (THF) and stirring for 10 min at room temperature. Dimethyl 3,3'-thiodipropionate (10.1015 g) dissolved in THF was added slowly dropwise. After dropwise addition, the reaction mixture was heated to reflux for about 1 hour. The reflux was continued for 1 hour after rinsing the dropping funnel with 10 ml of THF. The reaction was stopped and cooled to room temperature. The pH of the reaction solution was adjusted to 6-7 with 2% hydrochloric acid and then extracted with dichloromethane (30 ml x 3). The organic layers were combined, washed with saturated sodium chloride solution, collected and dried over anhydrous sodium sulfate. After filtration, the solvent was removed under reduced pressure to give 7.5639 g of a yellow oily liquid product in 88.7% yield.

4131-74-2
149 suppliers
$6.00/25g

4160-61-6
87 suppliers
$36.00/250mg
Yield:4160-61-6 99.9%
Reaction Conditions:
with sodium hydride in tetrahydrofuran;
Steps:
F Step F Synthesis of 4-Oxotetrahydro-2-indole-thiopyran-3-carboxylic acid methyl ester (a1)
2.7098 g of NaH (60%) was added to a 250 mL dry three-necked flask, and 40 mL of anhydrous tetrahydrofuran (THF) was added and stirred at room temperature for 10 min.Then, a solution of dimethyl 3,3'-thiodipropionate (10.1015 g, 0.049 mol) in THF (30 mL) was slowly added dropwise, and the addition was completed in about 1 h.Then, 10 mL of THF was added to rinse the dropping funnel, and the reaction was continued for 1-2 hours, and the reaction was completed. The reaction solution was adjusted to pH 6-7 with 2% dilute hydrochloric acid, then extracted with dichloromethane (50 mL*3), and the organic layer was combined and washed with saturated sodium chloride solution (50 mL*3), and the organic layer was collected and added. The sodium sulfate is dried and filtered.The filtrate was decompressed to remove the solvent.Yellow oily liquid 8.52,Yield 99.9%
References:
CN109280048,2019,A Location in patent:Paragraph 0056; 0093; 0094